Research - (2023) Volume 12, Issue 1
Received: 19-Jan-2023, Manuscript No. MBL-23-87389;
Editor assigned: 21-Jan-2023, Pre QC No. P-87389;
Reviewed: 02-Feb-2023, QC No. Q-87389;
Revised: 08-Feb-2023, Manuscript No. R-87389;
Published:
15-Feb-2023
, DOI: 10.37421/2168-9547.2023.12.361
Citation: Abbas, Fatima Musbah, Zehbah Ali Al Ahmad, Rehab Omer Elnour Elgezouly and Abubaker Elsheikh Abdelrahman. “Activated Carbon-Based Date Palm Leaves (Phoenix dactylifera L.), Prepared by Chemical Activations: TGA and DTGA Analyses, Young’s Modulus, Porosity and Electrical Conductivity.” Mol Bio 12 (2023): 361.
Copyright: © 2023 Abbas FM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Activated carbon pellets (ACPs), precursor (palm leaves) (Phoenix dactylifera L.), are primarily heated at low temperatures and milled into a fine grain powder to produce self-adhesive properties. The grain powders produced were impregnated in KOH solutions having a concentration of 0–0.35 moles (M) and pelletized by applying 12 metric tons of pressure, before being carbonized at 700°C, in a nitrogen environment. The thermal gravimetric analysis (TGA) and differential thermal gravimetric analysis (DTGA) were carried out on the pre-carbonized date palm leaves. The results showed that with increasing KOH concentration, the weight loss of the sample markedly decreased. The young modulus (E) showed that 0.25 M has a higher value of 10.6 GPs than the others, indicating better grain particle alignment. The porosity (B) of the AC was estimated empirically by adjusting the values of B to have a straight line with a slope of ≈ 2. The results found that the porosities of the AC fluctuated in a range of 0.39–48 with the KOH concentration. The electrical conductivity (σ) of the AC showed that 0.2 M was a higher value than the others, indicating that more mobility carried in the sample. The percolation theory was found to have a good correlation with the bulk density of the carbon samples, and this was interpreted by the increase in the bulk density of the AC sample above the critical density of 0.45 g/cm3 for the E and above the critical density of 0.045 g/cm3.
Date palm leaves • Activated carbon pellets • KOH • Young’s modulus • Porosity • Electrical conductivity
Date palm leaves (Phoenix dactylifera L.), waste material classified as lignocellulose material, composited of cellulose, hemicellulose and lignin, as the main products have several advantages, such as abundance, a low cost and an ability to be produced in large amounts. Traditionally, it is made into baskets, fans, crates, boxes, hats and fuel. It has been used as a starting material for the preparation of activated carbon because of its high carbon and low-ash contents. Thermal analysis of the carbonaceous material has been widely used to analyze the weight changes due to thermal decomposition during the carbonization process as a function of heat, temperature and environmental conduction [1,2]. Activated carbon materials are important in a wide range of applications, such as wastewater treatment [3] electrochemical capacitor and double layer capacitor [4-6], catalyst and catalyst support [7] and higher enegy storage [8]. Most of the work done has been made to improve activated carbon material such as the pore structure and surface are by pressure physical activation method [9] and self-adhesive and mechanical properties [10]. The pore size distribution, particularly the pore size distribution and surface chemistry of porous can be controlled by selecting suitable precursor and method of activation [4]. Therefore, chemical activation is widely used to prepare activated carbon, which involves mixing a carbonaceous material with dehydrating agents to facilitate surface properties at relatively stable temperatures, reported by many researchers [11-14]. It has been found that KOH activation is effective in enhancing the pore structure in a sample during the carbonization of precursors such as sewage sludge [15], sugarcane molasses [16] and rubber seed-shells [6]. In addition KOH activation reduced the weight loss at a higher concentration for the activated carbon from cotton cellulose during the carbonization process [2].
Mechanical and electrical properties are important for the application of activated carbon materials such as their use in electrodes [5,6]. The elastic modulus and electrical conductivity are widely correlated with the porosity in the porous medium [17,18]. So far, percolation theory has been applied into research to describe the behavior of a network modeling of the mechanical and electrical properties and its applications are discussed by many researchers [19-21]. It also facilitates the understanding of networked systems, such as robustness, epidemic spreading, vital node identification and community detection [20-22]. Also, there are many factors that play a role in determining the electrical conductivity (σ) of carbon materials, such as the porosity, moisture and extractable matter, all of which may inhibit or disrupt the electron flow. Frequently, it requires careful preparation to improve its physical and electrical properties [23,10,24].
In the present work, the Young's modulus of the AC was measured by ultrasonic techniques and correlated with porosity using mechanical modeling [21]. Electrical conductive was calculated from four point probe and the data produced were analyzed in terms of percolation theory. Date palm leaves were used as starting material to prepare activated carbon pellets, primarily pre-carbonized at low temperature and then milled into fine grain powder to produce self-adhesive properties. The objectives of this work are to carry out the thermal analysis on the DPL, untreated and treated grain powder for the grain and carbon pellets. Finally, the activated carbon pellets were characterized in terms of Young’s modulus, porosity and electrical conductivity and examined the effect of KOH activation.
Sample preparation
Palm leaves (Phoenix dactylifera L.) were collected from date palm trees growing near rural areas, urban areas and cities in southern Saudi Arabia. The date palm leaves were washed thoroughly to remove dust and other contamination and dried at 100°C. Cut them to a small size and precarbonized in a vacuum chamber at 280°C for four hours to cause them to shrink and break the palm leaves microstructure and release gases and non-carbon content [25]. The grain power produced is stored in a clean, self-sealing plastic bag in silica gel until used. Then the grain powder was impregnated with KOH having a concentration of (0.00 -0.35 moles), followed by stirring for two hours until mixed and finally kept in the mixture for 16 hours and dried in an oven at 100°C for 24 hours. The dried mixtures were further milled for five hours. About 2 g of each mixture was pelletized as a grain pellet by applying 12 metric tons of pressure in a mold of 2.75 cm in diameter and 9.5 cm in length. All grain pellets that exhibited an excellent binding property were characterized by bulk density and ultrasonic techniques before being carbonized at 700°C in a nitrogen environment (Vulcan Box Furnace 3- 1750), using a multi-step heating, scheduled as following: the heating profile was started at 1°C/min from room temperature to 375°C, where it was held for 1 h before heating was resumed at 3°C/min to 700°C, where it was finally held for 5 min. The system was automatically allowed to cool down naturally to room temperature. The carbon pellets were thoroughly washed with dilute H2SO4 and hot distilled water until a pH of 5 then dried in an oven at 100oC for four hours. Measurements of the grain pellets' and activated carbon pellets' dimensions were carried out using a micrometer. The bulk density was determined by dividing the weight of the sample by its volume. The bulk density was the overall density, inclusive of solid carbon and pore spaces. Results are given as the average of 5 replicates of each piece and examined as a function of KOH concentration.
The thermal gravimetric analyzer (TGA) and differential thermal gravimetric analyzer (DTGA) of palm leaves, PCDPL untreated and treated gain powder were measured using a thermo gravimetric analyzer (TGA50, Shimadzu) at 600ºC, in a nitrogen environment. The longitudinal velocity (V) of activated carbon pellets was determined using an Ultrasonic Pulsar-Receiver (Model 500PR) operating at 25 MHZ and equipped with PICO ADC-200 software. The pulsar division produces electrical pulses converted into ultrasonic signals using identical transducers. Petroleum jelly was applied as a coupling medium at the interface of the probe-sample. A reference glassy carbon Sigradur-(K) (CIG-K) was used to calibrate the ultrasonic signal and measure the samples' longitudinal velocity and elastic modulus. The measure Sigradur-K (SIG- K) agreed with the value given by the supplier with an error of < 1%. Then the Young’s modulus (E) of the one-dimensional form of the wave equation in a weakly attenuation region is given by Fatima MA, et al. [26]:
E =ρV2 (1)
Where ρ is the bulk density and V is the longitudinal velocity of the ACP. The elastic modulus (E) as a function of porosity (B) for bulk material was reported by many researchers [21] given as the ultimate analysis was estimated using the Equations (2) and (3) as:
E = E0 C (1-B)n (2)
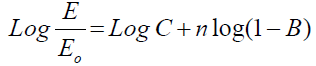 (3)
(3)
Where Eo is the elastic modulus for nonporous carbon material and n is the power exponential equal 2. The measurement was carried out by plotting log σ versus log (E/Eo) versus by fitting of B until a best linear relationship fit is obtained with the slope of n and the cut axis equal log C. In addition the E were analyzed in terms of the percolation theory threshold above critical density (ρc) [21] (equation 4) by plotting Log E versus Log (ρ-ρc) it gives straight line with slope of n (Equation 5)
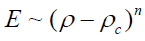 (4)
(4)
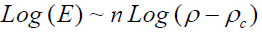 (5)
(5)
DC electrical conductivity (σ) was carried out on the CPs treated with 0.08 to 0.35 M KOH as following: First, the carbon samples were polished both sides, second, the I-V characteristic was measured using the four-pointprobe equipment (Keithley Micro-Ohmmeter) and the electric conductivity (σ) given as:
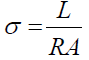 (6)
(6)
Where L is the sample thickness, R (Ω) is the electric resistance and A is the area of the sample. The electrical conductivity of carbon glassy SIG-K was used as the standard value to verify the accuracy of the measurement. In addition the electrical conductivity (σ) was analyzed in terms of the percolation theory threshold above critical density (ρc) [17] as:
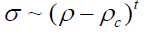 (7)
(7)
Where, ρc is the critical density. In this case, they reported that plotting of log σ versus log (ρ-ρc) for ρ>ρc for various values of ρc leads in every case to a straight line, with the slope, t, close to.
During the carbonization of the grain pellets, a large weight and volume change occurred with the body physically shrinking to maintain its structural integrity. The weight loss of the grain pellets during this process is expected due to the loss of non-carbon content and volatile compounds [25]. The result of the losses was that all the ACps evaluated had a reduced bulk density.
Thermal analysis
The typical DTA-curves of DPLs, untreated grain powder and treated grain powders with KOH are given in Figure 1 and summarized in Table 1. Initially, the decomposition process took place between 49 and 100oC, with moisture loss. Then, a strong exothermic reaction begins at about 250ºC with the weight loss reaching a maximum at 365ºC as the DPL's component decomposes. Similar results have been reported for the pyrolysis of lignocelluloses materials [25] and cellulose [27]. There was also little change in the decomposing temperature when the pre-carbonized carbon was treated from 0.0 to 0.10 M KOH, while it decreased gradually to 0.1 M KOH. In this case, decomposing KOH seemed to be having a catalytic action on lignocelluloses decompose in the palm leaves, thus lowering the temperature required. This argument supports the general notion that increasing the carbonization temperature reduces the amount of unstable volatile components in the sample [28]. Similar observations of cotton cellulose treated with KOH [2] and oil palm empty fruit bunches treated with H2SO4 [1]. The TGA data also shows that the weight loss varied with the KOH concentration and carbonization temperature used. Since all the pellets activated with KOH were prepared at the same compression pressure and carbonization temperature, the difference in weight loss can only be due to the difference in KOH concentration, with lower weight loss with higher concentrations of 0.35 (M) used as shown in Figure 2.
The DTGA data was displayed in Figure 3 shows two sets of peaks that represent a rapid rate of weight loss in the temperature range from 60oC to 120oC and from 270oC to 370oC respectively. The rapid change in the first peak range is associated with water and moisture evaporation from the samples and the results indicate that the treatment has enhanced weight loss. The peaks in the second represent a rapid rate of change in the precarbonized DPLs' decomposition or range thermal degradation of the date palm leaf structure. The treatment seems to shift the position of these peaks to slightly lower temperatures, as illustrated in Figure 2 and produces the overall trend that the height of these peaks is higher for the KOH treatment. Therefore, these data seem to reveal that the KOH treatment can promote the thermal decomposition of the pre-heated DPLs to occur at slightly lower temperatures and at a more rapid rate (Table 1 and Figures 1-3).
| KOH (M) |
Peak Temperature (°C) | Peak Temperature(°C) |
|---|---|---|
| DPL | 49.26 | - |
| 0.00 | 63.39 | 358.36 |
| 0.08 | 81.39 | 358.36 |
| 0.10 | 72.90 | 358.36 |
| 0.1.5 | 58.85 | 353.30 |
| 0.20 | 72.90 | 348.55 |
| 0.25 | 67.89 | 348.55 |
| 0.30 | 67.89 | 344.46 |
| 0.35 | 63.39 | 344.46 |
Bulk density and young’s modulus
During the carbonization of the green pellets, a large weight and volume change occurred, with the body physically shrinking to maintain its structural integrity [25]. This indicated that the grain pellets were subjected to compressive stresses during the heating process. Table 2 and Figure 4 show the average Young’s modulus (E1) and (E2) for the grain pellets and activated carbon pellets treated with KOH having a concentration of (0.08–0.35 M), respectively. Above 0.08 M to 0.15 M, the density increased rapidly and then decreased with a higher concentration of KOH. Thus, KOH concentrations of (0.0 M -0.15 M) are required to produce dense carbon pellets and reduced further above 0.15 M. Figure 5, shows that the elastic modulus of the carbon pellets increased slightly from 5.35 GPa to 10.53 GPa with KOH activation of (0.0 M - 0.35 M) E, increased with increasing KOH concentration, reaching its maximum at 0.25 M KOH of 10.53 GPa, indicating that the internal grain particles are squeezed together with less porosity. 0.25 M activated represents the optimal activation that can be used to activate the grain powder. Above 0.25 M KOH, the E decreased, possibly due to increasing porosity. Additionally, milling processes fine grain powder cased to transfer the raw material to more amorphous content in the grain powder and the grain pellets could be responsible for the mechanical reduction [29] (Table 2, Figures 4 and 5).
| KOH (M) | Grain Pellets | Activated Carbon pellets | ||||
|---|---|---|---|---|---|---|
| ρ1 | E1 | V | ρ2 | E2 | E2/E1 | |
| (Kgm-3) | (GPa) | (ms-1) | (Kgm-3) | (GPa) | ||
| 0.00 | 1380 | 2.73 | 2374.5 | 0940 | 05.35 | 1.96 |
| 0.08 | 1375 | 4.00 | 2285.4 | 1210 | 7.21 | 1.80 |
| 0.09 | 1346 | 4.42 | 2255.6 | 1283 | 8.76 | 1.98 |
| 0.10 | 1364 | 4.78 | 2748.3 | 1320 | 9.51 | 1.99 |
| 0.15 | 1387 | 5.75 | 2831.5 | 1271 | 8.44 | 1.47 |
| 0.20 | 1386 | 5.65 | 2674.1 | 1260 | 8.21 | 1.45 |
| 0.25 | 1388 | 4.90 | 2794.9 | 1348 | 10.15 | 2.07 |
| 0.30 | 1351 | 3.90 | 2755.3 | 1308 | 9.24 | 2.37 |
| 0.35 | 1348 | 3.05 | 2396.2 | 1200 | 7.03 | 2.30 |
| A | 1.250 | - | - | 1.180 | - | - |
| B | 1.290 | - | - | 1.230 | - | - |
| Sigradur Kb | - | 1540 | 35.0 | - | ||
| Sigradur Kc | - | 1540 | 34.9 | - | ||
Porosity
The porosity of the ACPs is estimated by adjusting fits (B) until a good straight line fit fits. First, for the classy carbon (Eo = 35GPa) and second, for the pure graphite Eo = 27 GPa. The plot of Log E/Eo versus (1- B) by adjusting the values of B until give a straight line with slope of n = 2.09 and cutting axis of -1.73 and -1.70, respectively as shown in Figures 6 and 7 and the fitting equations are given in equations (8 and 9). Both fitting data give similar power exponential n of 2.09, which is in good agreement with the review value reported previously (n = 2) [21] for three dimensional. The fitting data are presented in Table 3, showing that the B are in the range of (0.39-0.46) (Table 3, Figures 6 and 7).
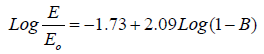 (8)
(8)
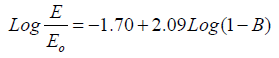 (9)
(9)
| KOH | Eo=35 GPa (Glassy Carbon SIG-K) | Eo=27 GPa (Pure graphite) | |||||
|---|---|---|---|---|---|---|---|
| Moles | E(GPa) | Log (E/Eo) | Porosity (B) |
1-B | Log (E/Eo) | 1-B | Porosity (B) |
| 0.08 | 7.21 | 0.8582 | 0.5 | 0.5 | -0.57343 | 0.46 | 0.46 |
| 0.09 | 8.76 | 0.93931 | 0.46 | 0.54 | -0.048886 | 0.42 | 0.42 |
| 0.10 | 9.51 | 0.97809 | 0.45 | 0.55 | -0.45318 | 0.41 | 0.41 |
| 0.15 | 8.44 | 0.92641 | 0.47 | 0.53 | -0.50502 | 0.43 | 0.43 |
| 0.20 | 8.21 | 0.91444 | 0.47 | 0.53 | -0.51702 | 0.43 | 0.43 |
| 0.25 | 10.15 | 1.00642 | 0.43 | 0.57 | -0.42490 | 0.39 | 0.39 |
| 0.30 | 9.24 | 0.9657 | 0.45 | 0.55 | -0.46569 | 0.41 | 0.41 |
| 0.35 | 7.03 | 0.84665 | 0.51 | 0.49 | -0.58441 | 0.47 | 0.47 |
The E based on the percolation theory, corresponding to the ACPs whose densities are located above its critical density (ρ > ρc) by fitting Log E versus Log (ρ –ρc) is displayed in Figure 8 and the fitting Equation (10). These results indicating that the mathematical model of E as a function of porosity and the percolation theory in terms of the bulk density is strongly depend on the power exponential (Figure 8).
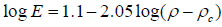 (10)
(10)
Electrical conductivity (σ)
The electrical conductivity of the CPs versus KOH concentration (0.08 M-0.35 M) is summarized in Table 4 and Figure 9. This shows that a sharp rise in electrical conductivity of the CPs is found to occur from 0.08 M to 0.2 M, because of the small amount of electrical contact between conducting microcrystalline. A similar observation was shown in the other carbon anthracites [30], which indicates that the carbonization temperature at which the charge carriers formed was able to penetrate the granular structure of carbon samples of more free carriers are created. This result is in the agreement with Kim DY, et al. [31]. The results also sample tends to have an electrical conductivity due to the inter-particles bonding of the sample improved by pelletizing pressure and or carbonization process. The Table 4 shows that the CPs from the treated grain powder has a considerably lower electrical conductivity than that of classy carbon (SIG-K). The data also show that the electrical conductivity slightly increased with the increasing KOH of (0.08 M- 0.2 M). KOH activation of 0.20 M exhibits a higher electrical conductivity than the others. Justifying, a better grain microstructure and small porosity that aid to more mobility carrier in the sample (Table 4 and Figure 9).
| KOH (M) | ρ (g / cm3) | ρ(Ω.cm)-1 | Log | (g / cm3) | Log (ρ − ρc) |
|---|---|---|---|---|---|
| 0.08 | 1.2068±0.04 | 2.733 | 0.4444 | 0.045 | 0.490 |
| 0.09 | 1.2837±0.11 | 2.912 | 0.4642 | 0.045 | 0.434 |
| 0.10 | 1.3198±0.10 | 3.063 | 0.0861 | 0.045 | 0.407 |
| 0.15 | 1.2701±0.13 | 3.451 | 0.5379 | 0.045 | 0.443 |
| 0.20 | 1.2611±0.5 | 3.471 | 0.5405 | 0.045 | 0.451 |
| 0.25 | 1.3470±0.23 | 3.216 | 0.5073 | 0.045 | 0.387 |
| 0.30 | 1.3176±0.31 | 2.978 | 0.4739 | 0.045 | 0.415 |
| 0.35 | 1.2001±0.021 | 2.949 | 0.4697 | 0.045 | 0.498 |
| C | 0.050 | ||||
| D | 02.460 | ||||
| SIG-Ka | 26.000 | ||||
| SIG-Kb | 23.000 |
Percolation theory
The electrical conductivity is based on percolation theory, corresponding to the ACPs whose densities are located above its critical density (ρ >ρc) by fitting log σ versus log (ρ – ρc) as shown in Figure 10. This fitting was made by varying the values of ρc until the best linear curve fit is obtained, with the slope of 2.12 to agree with the accepted universal value of t ~ 2 [17]. The intercept at the y-axis is 0.634 and the fitting value of ρc was found to be 0.045 g/cm3, which represents the percolation threshold (critical density) for ACPs samples. A similar observations have been found for the critical density of the anthracite carbon of (0.05 g/cm3), treated as a function of heat treatment temperature [32]. This value also reasonably two times of that for solid carbon pellets from date palm leaves of (0.025 g/cm3) treated with a different compression pressure [33]. However, the percolation theory was found to have a good correlation with the bulk density of the carbon samples and this was interpreted by the increase in the bulk density of the samples above 0.045 g/cm3. These results confirm that the variations of electrical conductivity for ACPs as a function of KOH treatment can be described in terms of percolation transition (Figure 10).
Activated carbon pellets (ACPs) from date palm leaves (DPLs) and their properties are satisfactorily tackled in this investigation. Fine grain powder, KOH activation and multi-step heating profile up to 700°C, as well as pellet form play a central role in improving the elastic modulus and electronic conductivity. 0.25 M KOH activation exhibit a higher Young’s modulus, while 0.2 M KOH activation is a higher electrical conductivity in the ACPs produced. The porosity contents were estimated from Young's modulus in terms of mathematical modeling and the electrical conductivities were characterized by a percolation theory threshold and the value of critical density was found to be 0.045 g/cm3.
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through General Research Project under grant number (GRO/323/43-1443) and acknowledge to Act Centre for great support.
None.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Molecular Biology: Open Access received 607 citations as per Google Scholar report