Research Article - (2024) Volume 15, Issue 5
Received: 01-Feb-2023, Manuscript No. JFR-23-88383;
Editor assigned: 03-Feb-2023, Pre QC No. JFR-23-88383 (PQ);
Reviewed: 21-Feb-2023, QC No. JFR-23-88383;
Revised: 21-Apr-2023, Manuscript No. JFR-23-88383 (R);
Published:
24-Apr-2023
, DOI: 10.37421/2157-7145.2024.15.556
Citation: Kaur, Simranjit, Himanshu Chauhan, Simran
and Saurav Kumar, et al. "Analysis of Unusual Trace Evidence-Paint
and Glitter." J Forensic Res 14 (2023): 556.
Copyright: © 2023 Kaur S, et al. This is an open-access article distributed under the terms of the creative commons attribution license which permits unrestricted
use, distribution and reproduction in any medium, provided the original author and source are credited.
Trace evidence are types evidence evolved when an object comes in contact with a surface (based on Locard’s exchange principle). Locard’s exchange principle states that: Every contact leaves its trace”. Whenever two objects comes in contact there is transfer of substances between them. They are microscopic in nature because it is difficult to detect to our naked eye. These types of evidence are crucial for the investigation and also help in reconstruction of the crime scene. Unusual trace evidence is a unique set of evidence found in the crime scene that will be play an important role in investigation. Trace evidence refers to minimal amount of sample particularly fibres, glass, hair, fingerprints, saliva, paint chips, glitter etc. The trace evidence presence depends on persistence of the evidence. The extent of persistence of evidence depends on size and shape, amount deposited, environmental factors and time.
Paint is a pigmented liquid composed of pigment, binder, liquid and additives. Used for protecting, decoration and for providing texture. Paint chips are mainly encountered in cases of hit and run, burglary, kidnapping, sexual assault and homicide. Paint evidence comes under two main categories class and individual. Class characteristics can be examined through chemical analysis of each layer as the manufacturer uses different combinations.
The paint analysis is performed in three forms they are mechanical, physical, and chemical. The mechanical involves by making a physical matches, chemical involves by determining the chemical composition and finally physical which relates to the color, texture, pattern and appearance. The paint from a vehicle can be traced based on specific modal and make as the manufacturer will mix different constituents with a specific formula which helps in curbing the suspect. Here in this project, we have performed the paint examination based on the following: physical examination, microscopic examination, solubility test and instrumentations.
Glitter as a trace evidence is found in cases of sexual assault, robbery, kidnapping, and accidental cases. It is collected by using a cello tape or post it- notes. The characterization of glitter is performed by using various techniques such as stereomicroscopy, FT-IR and SEM/EDX. Glitter is analysed based on their color, shape, size, thickness and specific gravity etc.
Instrumentations • Stereomicroscopy • Evidence-Paint • UV radiation • Pearlescent paints
Paint is a pigmented liquid which is used to cover a surface and remain as a decorative coating. It is a dispersion of pigment in a solvent. Properties of paint include: good hiding power, colour, resistance, easy application and economical in cost [1,2].
Composition of paint
Vehicle: When exposed air the oil dries off. Hence it is called as drying oil. The composition of oil is unsaturated but when exposed to air it gets saturated. It has the capability to maintain the pigment and other components of paint in the form of suspension [3].
E.g. Dehydrated castor oil, linseed oil, fish oil and bleached oil.
Dries: Added to drying oil to speed up drying. It acts as a catalyst for the oxidation.
E.g. Naphthalene, resonates, linoleats and metals like lead, magnesium.
Base: A solid substance that forms the body of the paint. It consists of titanium oxide, white lead, red lead and iron oxide which help the paint to become harder, elastic and strong and also provides protection against moisture and crack as well as UV radiation.
Pigments: It is a colored material used in combination with other components to provide color. Most commonly used in powdered state.
Solvents (Thinner): Helps in decreasing the viscosity of the paint. Usually added to make it smooth, uniform and easy application.
Eg: Petroleum, spirit, turpentine and coal tar.
Extenders (Fillers): Substance added to increase the bulk volume. Eg: chalk, gypsum, barite, silica and magnesium silicate [4].
Based on physical state
Solvent-borne paints: Consists of solid components (pigments, additives and binders) dissolved in an organic solvent. It dries fast and consists of binders.
Water-borne paints: Consists of water as the major constituents. They have a long drying time due to very slow evaporation rate.
Water-borne paints based on water-soluble binders: Polymeric binders are dissolved and having a low molecular weight.
Water-borne paints based on polymeric dispersions (emulsions paints): It consists of high molecular weight of polymeric binders dissolved in water just like colloids.
High-solid paints: Consists of solid components dissolved in organic solvent.
Powder coating: Prepared from powdered resin those which are attracted by electrostatic force of attraction.
Radiation curable coating: Combination of prepolymer, monomer and additive which protects against UV radiation.
Based on functions
Paint: Pigmented, non -transparent, protective and thin layered coating.
Varnish: Semi-transparent or transparent coating which is a mixture of binder, solvent and additives that consists of small amount of coloured substances which gives a glossy finish.
Enamel: A hard protective coating.
Primer: Coating applied primarily to increase the binding of paint and give a protection to the substrate.
Forensic importance of paint chips
• Hit and run cases.
• Burglary.
• Sexual assault cases.
• Homicide.
• Kidnapping.
Here in this project we are dealing with automotive paint use dprotection and decoration.
Automotive paint is made of water based acrylic polyurethane enamel paint because of reduced environmental impact [5].
Automotive paint forms
• Liquid polyurethane paints.
• Spray.
• Powder or additives.
Types
• Removable: Give appearance to vehicle.
• Non-removable: For touch-ups and for painting automobile.
• Process: Water spray of high pressure is forced onto the body.
Body is dipped in electro-coat-paint operation (E coat) and applies high voltage. Body acts as the cathode and paint acts as anode, then the paint gets stick onto the body.
Various coatings
Primer: First coat applied it helps in providing a smooth surface by clearing out all the defects. Helps in easy application, to stick onto the surface and prevents corrosion.
Base coat: Helps in providing a visual colour effects and it is applied after the primer. It is divided into three:
Solid: No sparkle effect used in aircraft, equipment for construction and transportation vehicles.
Metallic paints: Provides a metallic look and it consists of aluminium chips which gives sparkling effects.
Pearlescent paints: Also referred to as pearl. To provide finish works to create a depth of colour and spark.
Mottling is the process by which the metallic and pearlescent are applied uniformly that provide a visual and dark spots.
Clear coat: Applied on top of base coat and it should be resistant against abrasion, chemically stable and withstand UV light. It is solvent borne. The coating applied to the metallic body is 1 K formulations (one part) and to the plastic bodies 2 k formulations (two parts) are commonly used.
Collection and preservation
• Hit and run cases.
• Paint chips should be removed using a blade such as scalpel,
knife.
• Through the paint layers the blade must be cut down up to the
substrate and also run across it with pressure.
• Standard sample should be collected near the area of impact.
• Clothing or shoes found should be dried and packaged in a
paper.
Burglary cases
• Tool mark impression should be packaged carefully by not
placing the tool onto the impression.
• The whole tool should be submitted by wrapping them in a paper
to avoid contamination.
Packaging
• Items which are small should be packed in a paper and then
place it in a pill box by labelling the evidence.
• Items which are large can be placed in appropriate size plastic or
paper bags.
• Large items with sharp ends can be placed in a cardboard box
[6].
• The package must be labelled with appropriate case number,
item number and source of sample.
Analysis of paint chips
Physical examination: Colour, size number of layers, texture and state of the evidence.
Microscopic examination
• Used to determine the number of layers, sequence of layers,
surface markings and distribution of pigments is noted which
is done by using stereomicroscope.
• Microscopic analysis of a paint sample includes its
physical examination and its original physical condition that
includes its shape, size, color, texture and general condition.
• Microscopic analysis also includes minute details like number of layers in paint chip samples and their arrangement. It also
helps us to determine about extra layers coated over original
layers [7].
• Microscope that is used for microscopic analysis of paint chip
is known as stereomicroscope.
Stereomicroscope
• It is a type of microscope used for observation of sample under
low magnification power.
• It utilizes optical light which is reflected back from sample rather
than transmitted light.
• Stereomicroscope has 2 separate optical pathways; it contains 2
objective lenses and 1 eye piece.
• The arrangement of 2 objectives lenses and 1 eyepiece produced
3-D visualizations of the samples being examined.
• It is used to study the surface of a sample.
• It has two types of Magnifications one is Primary and the other
one is Pancratic.
Chemical tests
Solvent test and micro chemical test
• The samples to be tested are placed on a spot test plate and
prepare the sample by cutting a cross section. To the sample add
a drop of chloroform and observe the reaction under microscope
[8].
• Similarly add a drop of acetone. The same sample can be used if
the sample used along with chloroform does not react
significantly.
• Other chemicals used are: Conc H2SO4, conc HNO3, conc
HCL, conc NaOH, Ammonium sulphide, ethyl acetate, Le Rosen
reagent (37% formaldehyde 10 drops in 10 ml of sulphuric acid).
Solubility test and TLC
• The solubility of paint is tested using various chemicals and the
components which are soluble, examined by thin layer
chromatography.
• Microchemical test: Test in which various ranges of chemicals
are added which is used to determine the composition of paint.
• Chemicals: Acetone, ethyl acetate, chloroform, benzene,
methanol, methylene chloride, and dimethyl form amide and
xylene [9].
Instrumentations
Fourier Transform Infrared (Ftir)
Principle: At different frequency the elements absorb light at different wavelength. It creates molecular fingerprints of the sample.
Uses: The unknown material can be identified, quality of the sample and the elemental composition.
The molecular fingerprints give off the peaks of absorption which represents the vibrational frequency in between the bonds. Since the suspected samples have different combination of atoms which gives unique spectrum [9]. Hence it helps in unique identification of the sample and also the peak size determines the amount of material.
Analysis of sample
• Source: The source emits infrared radiation from a black body
which passes through aperture.
• Interferometer: Beam after passing the aperture goes into the
interferometer and coding of spectral beam occurs. The resultant
beam emerges out as the interoferogram.
• Sample: The interoferogram is reflected or transmitted through
the sample that is being analysed. Here certain frequency of
energy is absorbed by the sample.
• Detector: The interferogram signal is detected.
• Computer: The Fourier transformed signal which is digitized and
the interpretation of results take place. Measurement of
• Background spectrum: It is measured without a sample in
between the beam and compared without a sample in between
the beam gives the percent of transmittance which helps in
overcoming the instrumental defects.
Advantages
• Speed: Different frequencies can be measured simultaneously
within seconds. Also called as felgett advantage.
• Sensitivity: The optical throughput is high (Jacquinot advantage)
and low noise levels as well as fast scanning by adding more
scans to reduce the noise(signal averaging).
• Mechanical simplicity: The breakdown is prevented
mechanically with the help of moving mirror in the interferometer.
• Internally calibrated: It has HeNe laser which is self-calibrating.
• Helps in positive identification of the sample and assured quality.
Disadvantage
• Single atomic species cannot be detected.
• Two identical atomic which are symmetrical cannot be detected.
• Difficulty in examination of aqueous solutions.
• Samples containing complex mixture give rise to complex
spectrum.
Pyrolysis gas chromatography
Principle: Under an inert atmosphere the sample is heated at high temperature which is degraded into smaller molecules which is divided into various components by gas chromatography and confirmed by mass spectroscopy [10,11].
An analytical column is kept in between the pyrolyzer equipped with GC through an injection port. Components get separated when the inert gas such as nitrogen or helium is flowed across the sample. The components are detected using mass spectroscopy.
Types of pyrolyzers
• Micro furnace: The sample temperature is raised continuously
till the pyrolysis temperature.
• Curie-point pyrolyzers: Induction coil is heated in which the
sample is loaded at one end of the pyrolysis wire that consists of
ferromagnetic alloy placed into the pyrolyzer. Temperature keep
on increasing until the Curie point is reached and the magnetic
property is lost gradually. Temperature remains constant at this
stage till the magnetic coil is switched off.
• Filament pyrolyzers: The sample is placed in a direct contact
with the wire coil in a tube and it is heated till a particular
temperature, conduction of heat takes place which causes the
sample to get pyrolyzed [12].
Working
Magnetic fields can deflect atoms and molecules; through which atoms are firstly changed into an ion. Magnetic Field affects electrically charged particle, but not electrically neutral particles.
Ionization: Knocking electrons give rise to a positive ion, hence ions and molecules get ionized.
This is also applicable only for those ions which form negative ions and also for those which never form ions. The mass spectrometer works only with positive ions.
Acceleration: Ions are accelerated from for having the same accelerated energy to ions.
Deflection: The ions are deflected according to their masses. Lighter molecules get deflected more. The ion which gets deflected is based on the negative charge on the ions.
Detection: based on the negative charge on the ions. The passing beams of ions are detected electrically.
Advantage
• It is a very simple process.
• Relatively inexpensive than other techniques.
• Capable of processing a wide variety of samples.
Disadvantage
• It is a destructive technique. Samples used for pyrolysis cannot
be used for any other analysis.
• Uneven heating of sample can spoil the results.
• Huge amount of heat is generated.
Applications
• Art forgery
• Evaluation of biological sample.
• Environmental.
• Detection of any adulterants.
Scanning electron microscope
Principle: The topography and the composition of the surface are obtained when the electron beam comes in contact with the sample and produces various kinds of signals [13].
Working components
• Electron source.
• Column through which the electron passes to reach the
electromagnetic lens.
• Detector.
• Sample chamber.
• Computer.
The energy is transferred to the sample when an electron beam from the incoming rays is bombarded. The electrons which are bombarded are referred to as primary electrons which eject the electron from the sample. The primary electron goes deep into the sample and release secondary electrons. These electrons are the fed to the detector and translated to signal. Apart from secondary electrons back-scattered electrons are produced from the primary electrons. It is difficult to analyse by the electron detector. SEM is attached with EDX to determine the elemental composition.
Applications
• Identification of firearm.
• Gunshot residue analysis.
• Paint examination.
• Handwriting examination.
• Counterfeit currency notes.
Advantage
• Three dimensional structures in detail.
• Surface imaging.
• Easy operation.
• User friendly.
• Faster working capacity.
• Data is generated in a digital form.
• Easy sample preparation.
Disadvantage
• Size and cost.
• Maintenance is difficult.
• Specialized training is required.
• Sample prepared might contain contaminants.
• It is applicable only for solid samples.
• Radiations those scattered beneath the surface get exposed.
SEM imaging
The electron gun generates a beam of electrons which passes down the column and then to the electromagnetic lens. The lens is folded in a tube of coil called as solenoids. Coil helps in adjusting the voltage that comes in contact with the specimen. The electron beam then falls onto the sample placed on the stage. Samples should be placed in a low pressure in vacuum. Incident electrons when come in contact with sample the secondary and backscattered electrons are generated which is detected by using various types of detectors [14,15].
Modern automotive coating process
There are five steps involved in the coating process:
Pre-treatment: It helps in forming a surface texture by removing and cleaning excess of metal that enables the bonding of the protection layer against corrosion.
Electro deposition: It helps in preventing the formation of rust.
A Poly Vinyl Chloride (PVC), a sealer: Which that is applied for preventing corrosion, for eliminating leakages caused due to water and also for minimizing chipping property and vibrations caused by noise.
Primer: The application of primer is performed, for enhancing the contact among the basecoat, surface which gives off a smooth surface and also minimize the process of chipping.
The various coatings such as topcoats, basecoat and clear coat are applied which provide color. Glossy finish, resistance to weather and smoothness [16].
Pre-treatment
The metal components are used to make the automobile body by welding them together and forms a structure called as body-in white. Pre-treatment involves cleaning the surface by removing the oils caused due to stamping process and welding residues is done primarily by three process degreasing, conditioning and phosphating.
Phosphating: A layer of phosphate is applied to provide resistance for corrosion.
Degreasing: It is a solution made up of surfactants and alkaline compounds such as caustic soda, sodium carbonate and trisodium phosphate. The surfactants involve various detergents on BIW.
There are two sequence which includes stage of spraying known as Knock-Off Degrease (KOD) and dip sequence which takes place in degreasing zone.
It helps in removing oils and dirt by high pressure spray and also provides fewer loads to the dip sequence.
The surface conditioning also referred to as activation that creates sites of nucleation, which helps in increasing the crystallization nuclei on metal surface for enhancing bonding.
Conditioning: Titanium orthophosphate is used.
Electro-deposition (ED)
For anti-corrosion body parts and frames made of metal are coated. When the coating is not done the metals are primed for applying the coatings-coat which is a combination of resin, binder and paste that consists of pigment and solvent. The anodic electrode position, metal surface is positively charged and paint negatively charged in which, ions migrate and creates a coating. In case of cathodic electrode position the metal surface is negatively charged and paint is positively charged.
The caustic and the acidic components in the ED tank that gives the measurement of pH, particular solvent used. The functional groups present in the resins as well as the neutralizing agents helps in maintaining a balance. A combination of resin, binder, paste and pigments are added into the ED tank where the automobile was dipped into ED tank and electric current is provided. The tank also consists of deionized water and paint solids. For the paint solids the deionized water main role is to remain as a carrier under agitation. Charged nature of the process of coating the ED goes deep into the places where a spray cannot reach.
Resin provides protection against corrosion, makes the paint tough and durable. Pigments function is to provide gloss and color. When an automobile is withdrawn from the tank the surface have paint adhering onto the surface; those which are not bound are rinsed off from the surface by ultrafiltration to enhance the smoothness.
Then the automobile body is being fed to the bake oven where the heating and temperature is around 160°C is maintained for 10 minutes which helps in increasing maximum performance. It helps in providing chip resistance, adhesion resistance and protects against corrosion. In some cases a water spots are present on the body surface from the conveyor belts or some other sources it happens when there is no proper deionization of water and due to higher conductivity. These spots of water on the surface causes problems in coating hence it should be removed by a process called as sanding and also by adding ultra filtrates or surfactants to the rinse zone [17].
A sealer: PVC (Rust proof material)
A sealer such as PVC or urethane is applied on the inner side of doors, front dash board, trunk, hood and exterior and interior metal joints and back wheel. It is either done manually or with helps of robots. Currently PVC or acrylic urethanes are used in areas of under body which is called as dampening coat or DC. It helps in providing noise proof and decreasing the vibration. The sealers reduce the vibrations and noise to the compartment of passengers and also the noise generated from the engines, suspension systems, noise from the tires when moving on the road and the flow of air. The sealers provide protection against corrosion, resistance to chipping which is primarily done by using airless sprayers or robots.
In the next step a soft tip primer is applied to the front hood edge where it is more susceptible to chipping, with the use of high elastic resin that is seen in between the electro-deposition and coats of primer.
The rear areas of body, radiator support and housing of wheels are coated with dull black pigment referred to as blackout coating [18].
Primer
The small imperfections created by sanding and grinding are being filled by primer. The function of primer is to level out all the imperfections and to give a smooth finish, protection from anti-corrosion helps in increasing the contact between primer and the E-coat also the basecoat. The primer layer helps in preventing the process of chipping, when the process of chipping reaches metallic surface or body it may lead to corrosion. It also helps in improving the contact of topcoat onto the primer surface which ensures outer finish when chipping takes place.
Hence the primer should be mixed with various colors in order to mask the damage onto the topcoat due to chipping.
The primer coting is done in three stages exterior coatings, interior coatings and then drying them in an oven. For coating the interior such as doors, compartment of engines and luggage space the manual spray method is used. To impart a uniform coating appearance the basecoat color should match with primer color. The body of the automobile is dried by passing it into an oven at a temperature 140 degree Celsius for 30 minutes.
Topcoat
Top coat consists of two types of layers: Base coat and clear coat.
Base coat consist of paint pigments and the clear coats provide protection against effects of environment, corrosion, resistance against UV light, provides smoothness and a glossy finish. Automobile body is firstly coated with basecoat and then a clear coat is applied before heating an oven at a temperature of about 30 to 40 minutes at 125°C [19].
Basecoat
The color put on the base coat is evaluated is based on certain standards terms, with hue and Chroma. Pigments are particulate solid particles insoluble in paint. The pigments are effective based on the aluminum flakes, mica and other interfering agents. Aluminum flake the lightness or darkness is based on an observation angle and the effect is referred to as lightness flop and mica the observation angle is referred to as color flop. When the concentration level of pigment varies the color intensity applied on the base coat also changes. Hence various finishes can be designed based on the hue and Chroma values.
Clear coat
Function: provide resistance against scratch prevents fading against UV rays and also helps easy repairing and maintenance works.
Environmental etch is a phenomenon in which the automobile coatings such as the clear coat is deformed by forming a water spots which are permanent or marks which not removable caused due to bird dropping, resins from tree or exposure to chemicals.
HALS (hindered amine light stabilizers) chemical in combination with ultraviolet light absorbers blocks the UV radiation by forming a backbone made of polymers and absorbs radiation between 290- 400 nm wavelengths.
Some scratch are seen on the clear coat only when it is exposed to scattering light and some might rise up and break into fragments. The characteristics such as flow depend on 1 K (no need of hardener or a catalyst for thickening) or 2 K (need to be used in combination with hardener or a catalyst for thickening). 1 k coats provide plastic flow resistance and 2 K provides fracture and resistance to impact.
The most commonly used 1 K is acrylic melamine, it is based on combining polyols of acrylic and agents of amino acids cross linking.
The 1 K and 2 K polyurethane provides resistance against environmental etch and scratch, and also 1 K and 2 K epoxy resins provide resistance against etch caused due to acids. Water borne clear coats based on the combination between polyester and acrylic cross linking with melamine and isocyanate. But the powder clear coats are used commonly because the environmental concerns (less emission of VOC). It also have other advantage such as the powder can be reused again, waste water is not produced during application, organic solvent is not used while applying the paint or for cleaning, the total energy supplied is reduced because the supplied air can be recycled, non-toxic and provides thickness which is uniform in appearance.
Spray coating
The spray coating on various coatings is done by using the atomizers. Spray is an immersion of droplets dissolved in gas phase that is condensed. Sparys are formed due to atomization which is generated by the result of propagation of droplets from the liquid by various forces such as electrostatic forces, mechanical and aerodynamics. Spray coating is done with help of various applicators such as air spray guns, rotating disk or bell i.e. both which are electrostatic or non-electrostatic [20].
Automatic spray coating is done by using robots hence it is called as robotic coating. Spray booths is required to perform the spray coating. The greatest advantage of spray coating is the efficiency of transfer to the target and also particles of the paint those which do not coat the body of the automobile and particles float in air.
Automotive spray applicators are of two types: Air spray guns and high-speed rotary bell.
Issues with automotive spray
The atomizer atomizes the paint into droplets and coats the surface of the automobile. The issues include: transfer efficiency, deposition of coating, consumption of energy, efficiency of engine, environmental and safety of work place. These can be analysed from the design of atomizers, composition of paint and modifications in the surface. The transfer efficiency is defined as the ratio of amount of paint that coats the surface to those amount ejected from the atomizer.
Transfer efficiency depends on factors such as:
• Characteristics of the target surface.
• The design of spray.
• Parameters of working.
• Conditions of air in the spray booths.
• Coating characteristics-liquid.
The paint which is over sprayed should be incarcerated before it entering the atmosphere, hence the method used to prevent to ptisca ure is the water washing or wet scrubbing. Overspray capturing efficiency referred to as the ratio of captured amount of overspray to the quantity which has entered the capturing system.
Trends in automotive coating process powder coating
The liquid coatings often used have larger emissions of volatile organic compounds; hence the use of particulate solids in the form of powder called as powder coatings reduces the emissions of VOC. It is used in the handles of doors, decorative systems, radiators, engines, filters, wheels, bumper etc. Powder coatings often provide a glossy finish and protection against corrosion on the any metal when applied.
Powder is mixed with smaller particles of pigments and resin and then the resultant is sprayed electrostatically onto the surface. Powder particles which are charged bind to the surface that is electrically charged, till the powder is heated and gets fused to the smooth surface coating when it is cured in the oven. Then it forms a high quality attractive finish coating.
Advantage of powder coating: Reduce production of VOC, overspray can be recycled, more transfer efficiency, provides thick coatings, low cost, special effects can be achieved when used in combination with powder coatings, shows lesser difference in appearance than liquid coated. Disadvantage of the powder coating is when it is used in combination with the metal particulates.
Wet paint
The waterborne 3 wet paint system in which the application of primer coat firstly then basecoat and at last clear coat then the coatings are cured in the oven. Hence this process is also called as 3 wet process. It is time consuming comparing to the rest and VOC generated is very less, low cost and environmental friendly. Disadvantage of this system is that surface of rough substrate and the primer and then water in basecoat might interfere in the primer layer. These are overcome by using improved steel, ED tank appearance and two stage bake use. Currently, water borne 3-wet paint system with qualities equal to three-coat or two bake was generated by using basic resins with low melamine content in paint helps in reducing the shrinking as well as for promoting the leveling.
Automobile manufacturers
Maruti Suzuki-1981: It is a joint venture of an Indian automobile company and a Japanese car and bike manufacturers formally known as MARUTI UDYOG LTD. 56.21% of the paint is owned by SUZUKI the rest is owned by the public sector of India. Together it has manufactured various popular cars such as Ciaz, Alto 800, Alto K 10, Ertiga, Eeco, Baleno, Ignis, Vitara Brezza, S-cross, Wagon R, Swift.
Mahindra group-1945: Founded by Jagdish chandra mahindra, Kailash Chandra mahindra and Malik Ghulam Mohmmad. Initially emerged as steel manufacturers. The present managing director Anand Mahindra came up with a new logo in 2000 and made Mahindra Scorpio the most successful among other entirely indigenously made four wheeler cars. The company has emerged as a big name with around 100 plants across the globe. Essentially it has shown presence in aerospace, agribusiness, automotive, insurance, farm equipment, and hotels defence and construction equipment.
Tata motors-1945: Founded by JRD Tata. Formally known as TATA Engineering and Locomotive Company Ltd (TELCO). TATA launched Indica in 1998 the first fully indigenous Indian Passenger car.
Tata motors have various working plants across the country at Jamshedpur, Sanand, Pune, Lucknow, and Patnagar and Dharwad. Globally it has plants at Great Britain, South Korea, Thailand, and Spain and South Africa.
The most remarkable event was the acquiring of Jaguar Land Rover, an English premium Car. The most famous cars are Indigo, Indica, Nano (the world’s cheapest car), and Sierra.
Honda-1948: Founded by Soichiro Honda and Takeo Fujisawa. It is a Japanese conglomerate and a lead manufacturer in motorcycles, automobiles and power equipment’s. In India it is headquartered at Greater Noida, Uttar Pradesh. It has joint venture with Indian automobile company hero motor corps owned by Munjal’s but announced split in 2010 selling its 26.78% stakes to Munjal’s. Popular models by Honda are: Honda Amaze, Honda City, Honda Civic, Honda Jazz, and Honda Accord.
Royal Enfield-1901: Founded by Albert Eadie and Robert Walker Smith. In 1955 it partnered with Madras motors a Chennai based Motorcycle Company and assembled the 350 cc Royal enfield bullet. The first product manufactured by Royal Enfield was a Quadricycle, a bike with four wheels. The most popular model was 250 cc bullet manufactured during World War II. The most popular models are Royal Enfield Classic 350 cc, Royal Enfield Thunderbird 350 X, and Royal Enfield Interceptor 650.
Toyota-1997: Founded by Kiichiro toyoda. It is a Japanese market leader in producing hybrid electric vehicles and hydrogen fuel vehicles in world. In India it is headquartered at Bidadi, Karnataka. In India it has a joint venture with Kirloskar Brother’s Ltd which is an Indian conglomerate India’s largest manufacturer of pumps and valves. The subsidiary is known as Toyota Kirloskar Motors headquartered at Bangalore, Karnataka.
89% of the stake held up by the Toyota group and the rest is owned by Kirloskar Brother’s Ltd. The most popular models are Fortuner, Camry, Qualis, Etios, Corolla, Innova, and Land Cruiser.
Ford motor company-1903: Founded by Henry Ford. It is an American Multinational Company that manufactures commercial vehicles and luxury cars under name Lincoln Brand. It ows 8% stakes British Luxury Vehicle Brand Aston Martin. Ford (49%) has joint venture with Mahindra and Mahindra Motor corps. The most popular cars nowadays are Figo, Ecosport, Mustang, and Endeavour.
Chevrolet-1911: Founded by Louis Chevrolet and William C Durant, it is an American Car manufacturer headed by General Motors. The only plant in India is at Talegaon which is an export only facility. It is the 5th largest name in car industry in India. The most popular cars in market are Beat, Cruze and Travera.
Joseph Cyril Bamford (JCB) excavator’s Ltd-1945: Founded by Joseph Cyril Bramford is a UK based company exclusively working in manufacturing machines for Digging, Tractors, Excavators. It is the lead producer and assures a quality of heavy Weighing Machines. Various Models are present nowadays such as JCB 3DX, JCB 3DX SUPER, JCB 3DXL, JCB 3DX XTRA with an average price range of 20 lakhs to 40 lakhs INR.
Fiat-1899: Founded by Giovanni Agnelli. It is the largest Italian automobile manufacturer and thirds largest In World. In India it is headquartered at Pune, Maharashtra. The most popular models nowadays are Punto, Jeep Compass, Palio, and Siena.
Glitter
Glitter is made up of tiny particle that shows the property of reflection when light is incident on it. Glitter is used in decorative articles, greeting cards, cosmetics, clothes, and ornaments etc.
These are available in different colors, size, and shape. These properties are attributed to the manufacture, how he wants it to be done.
Types of glitter
• Holographic glitter: These are the most radiant types of glitter
that reflect the incident light into many radiant colors. These are
used in nail arts, home decor articles etc.
• Metallic glitter: These glitters are not actually made up of
metals, but the word metal actually denotes its property to shine
like metal. These are the most commonly used for body art.
• Irridescent glitter: These are the glitter having soothing
appearance and available in various shades these are widely
used in cosmetic industries for preparing nail paints, lipsticks,
highlighters, and eye shadow etc.
Particle size
• Large size-0.5 mm-0.8 mm.
• Normal size-less than 0.2 mm (most commonly used).
Forensic importance of glitter: Glitter is trace evidence that is entirely man-made. The glitter can be of various shapes, sizes and colors that vary from manufacturer to manufacturer making it easier for the forensic expert to link it with its source.
Glitter can be recovered as evidence from various cases such as:
• "HIT and RUN” cases where glitter particles worn by the
suspect can be recovered from alleged vehicle.
• "RAPE and MURDER CASES” glitter obeys Locard’s principle
of exchange thus it can be mutually exchanged between
suspect and victim during the course of the incident.
“Kidnapping cases”: What makes Glitter ideal trace evidence?
• Nearly invisible.
• Easily transferable.
• Can tolerate extreme conditions.
• Maximum retention time.
• Variable shape and size.
• Easy sample collection and storage.
• Least degradation.
Forensic analysis of glitter
Collection: Glitter being highly reflective in nature can be located using flashlights.
It can be collected using adhesive tapes or post-it notes. And then can be packaged in zip-lock bags and sent to laboratory for further examination.
After successful separation and collection a forensic investigator would go for:
Physical analysis: Color of particles.
Shape and size of particles: Most commonly the glitter particles are hexagon, Square, Rectangular in shape. However other shapes such star, crescent, round etc. also nowadays manufactured.
Size of the particles is measured in millimetres where normal size range is less than 0.2 mm and size range more than 0.5 mm is considered large.
Thickness: Manufacturers around the world have confirmed the presence of metallic layers used in glitter. Coatings of aluminium, titanium dioxide, bismuth oxychlorides and iron oxides are often used. Thickness varies from 50-175 microns.
Specific gravity: Because glitter is coated with different metallic or plastic layers of different thickness, its specific gravity will vary accordingly.
However the normal specific gravity varies from 1.2-1.25.
Disadvantages of physical analysis
• Provides clue only of the class of the evidence.
• Changes of getting false positive results.
Instrumental analysis
ATR-FTIR micro spectroscopy: Attenuated total reflection- Fourier transform infrared spectroscopy is a sampling technique used for the analysis of reflectance either surface of glitter particle. The major advantage is that no prior sample preparation is required; the only requirement is that, either side of particle is free from dust.
Dispersive Raman microspectroscopy with confocal imaging: It is a non-destructive technique which can easily distinguish between various coatings on the glitter particle without any prior sample preparation. With confocal imaging analysis of even deepest layers can be done.
Scanning Electron Microscopy (SEM): It is a non-destructive technique that focuses a beam of electron on the sample to give information about the topography of the surface and the composition of the particle.
Magnetic levitation: It is used for measuring the density of magnetic substances when suspended in Para magnetic solution. Glitter being coated with metallic substances can be subjected to magnetic leviation method.
Disadvantages of instrumental analysis
• Not feasible.
• Expensive to use.
• Time taking and complex procedure.
• Magnetic leviation causes degradation of sample.
Glitter manufacturers in India
• Advance Syntax Ltd. (Vadodara).
• Global export and import (Jabalpur).
• Lakhotia Polyester India Pvt. Ltd. (Nashik).
• Rohvyo Impex (Mumbai).
• Polyester Marketing (Seccunderabad).
• Astar Foils (New Delhi).
Here we did analyses on three manufactures
• Middas: It is an Indian glitter manufacturer having the main office
at Vadodara Gujarat. They produce a fine quality of
aluminium glitter, polyester films, cosmetic glitter, textile glitter
powder, hot stamping foil, epoxy resins and glitter crafts for kids.
• Tenderberry.
• Vozwa.
Terminology
ATR-FTIR (Attenuated Total Reflection-Fourier Transform Infrared): Technique used to identify the composition of the given sample with the help of absorption peaks specific to certain functional groups.
SEM (Scanning Electron Microscope): Non-destructive technique that focuses a beam of electron on the sample to give information about the topography of the surface and the composition of particles.
TLC (Thin Layer Chromatography): Separation technique used to separate the constituents of sample due to interaction of particles of the sample with the mobile phase and stationary phase based upon which the respective Rf values are calculated.
Specific gravity or relative density: It is the measure of density of a material based upon the ratio between the densities of that substance to the ratio of the reference sample in most cases which is water.
UV rays (Ultra-Violet Rays): It is a part of electromagnetic spectra having wavelength of 10 nm-400 nm which lies between X-rays and Infrared rays.
ED (Electro-Deposition): Sometimes called as electroplating refers to the process of coating of a material onto to a conductible surface with the help of electronic charge.
Stereomicroscope: An optical instrument capable of providing a 3- dimensional image of the specimen with the help of reflecting light.
Multi-modal compositional analysis of layered paint chips of automobiles by the combined application of ATR-FTIR imaging Raman microspectrometry and SEM/EDX-MD Abdul Malek
The examination of paint chips are crucial for determining number of layers, painting process, paints used, type of automobile, color, texture and protection agents used for surface that depends on the manufacturers and model year of manufacturing. Optical microscope such as SEM/EDX, ATR-FTIR imaging provides information about the number of layers, sequence of layers, surface markings and thickness
• EDX-elemental analysis.
• ATR-FTIR-profiling or imaging molecular level of paint.
• RMS-inorganic fillers.
To determine physiochemical property and variability in each layer, the cross section of sample produces large amount of spectral data so knowledge on machine techniques is required for classification.
Inter-rater reliability of vehicle color perception for forensic intelligence-Khai lee
Top coat of vehicles provides lots of information in case of accidents. The witness of car accidents helps in curbing the suspect. The research paper mainly deals with color determination by volunteers and by comparing the outcome to prevent the wrongful convictions. The outcomes are of two types, a match or non-match. It follows statistical analysis based on comparison between stationary and moving vehicles.
Frequency of matches and inter–rater reliability depends on basic description of color, disregarding the shades. White and black showed greatest match but intermediate color was confusing. The information from this research paper helps in identifying the top coat color of vehicle based on witness statement which turns out to be more reliable.
Evolution of the automotive body coating process-Nelson K. Akafuah, Sadegh Poozesh, Ahmad Salaimeh, Gabriela Patrick, Kevin Lawler and Kozo Saito
The automotive coating technology has changed over time. The composition of coating and coating process are dependent on each other. The final coat which is applied it is a combination of different composition. The composition of coating, coating process and characteristics of surface will determine the coating film appearance. Automotive coatings face one of the major challenges such as the environmental contamination. The manufacture process coatings based on the customers interest or perception, hence it is unique to each manufactures. This paper clearly discuss about the modern technologies used in automotive coating process, recent trends, potential developments in future and composition each coatings.
Forensic examination of car paints-Jakub M. Milczarek
Paint chips are transferred to the victims clothing in case of an automobile accident and robbery. Two types of investigations are performed: Identification/classification and comparison. The paint basically consists of four types of layers: Primer, surface of primer, basecoat and clear coat. The techniques used for analysis are as follows:
• Microscopic examination.
• FT-IR.
• SEM/EDX all the techniques are explained in this paper in detail.
The paper ends by putting forward a suggestive work which includes setting up a database for car paint that will help in narrowing down the area of search more effectively.
Identification of tiny and thin smears of automotive paint following a traffic accident-Yun-Sen Giang
The paint chips are analysed by three techniques
• Stereomicroscopy.
• FT-IR.
• Solubility tests for determining the known and unknown sample.
The three methods used had its own limitations which caused a difficulty in comparison.
Exchange of paints in hit and run collisions (Ajay Kumar)
As we know that there are no eyewitnesses in hit-run cases, so physical evidences provide crucial information in crime cases which depends on the realizing, protecting, selecting, identifying and for evaluating of the transferred material paint. In the hit and run cases usually there is transfer of paint, between the suspect’s vehicles with that of the victim in the scene of crime. It is linked to accused vehicles with that of victim’s vehicles after collision.
The evidence recovered, helps in proving the suspects innocence. Paint sample are preserved until sample can be forensically examined. The main significance of this review paper will be paint as most important evidence in hit and run cases with the help of relevant case study. From the case study we will discuss about how it collected the paint sample from the crime scene and how it is preserved for the forensic examination. In many hit and run cases there is no eye witness at that time physical examination play an important role for solving the cases.
In this review paper we have mentioned the material physical evidence for example paint chips which can be useful in hit and run cases to identify the origin of the sample.
Glitter the ideal trace evidence, Richard Kam, Ml inda Xiao, Xanthe Spindler, Philip Maynard, Clau de Australia
According to the given research paper it was found out that glitter is entirely man-made has been used since ages for decorative purposes and in cosmetics. Small particles of glitter are cut out of large aluminium sheets into various shapes such as hexagon, square and rectangle. Nowadays the evidential value of glitter has been subsequently increased because of its increasing use in various products and also involved in various criminal cases as evidence.
Instrumental analysis
Raman micro spectroscopy: For the better analysis of inner layers of glitter particles.
Scanning electron microscopy/energy dispersive spectroscopy: For the elemental analysis of each particle.
Results and discussion: It was found out that there were no glitter manufacturer’s in Australia. Most of the manufacturers were from United States and China. Results were based upon size, shape and color. Color was the most undermining factor which can be affected by the light conditions, angle of observation and various coatings.
Out of the shapes hexagon was most common thus it had a very low evidential value. Other special shapes such as rectangle, heart and star shape were most uncommon thus it had a very high evidential value.
Conclusion: Glitter particles from different manufacturers were examined by optical, physical and chemical techniques.
FTIR was most differentiating technique due to its depth analysis of various chemical coatings. The results were compared with a computer database.
Scope of study
• Paint is a type of evidence obtained in case of hit and
run, robbery, burglary or breaking into house.
• Hence paint chips are examined at the scene of crime.
• The importance of paint chips as an evidence increases when
it is obtained from the vehicle of suspect in cases
accidents, clothing or footwear of suspect or from the house
under suspicion in cases of break-in.
• Paint is commonly obtained as flakes, powders or chips.
• To reveal the possible connection of transfer of paint chips from
one surface to another the forensic examination is conducted on
the suspected samples.
• Based on the law of comparison, a comparative study is
employed on the paint chips using different techniques
for identifying the origin of the sample from the same source or
not.
• Glitter is made of tiny particles that show the property of
reflection when light is incident on it.
• Used in decorative articles, greeting cards, cosmetics, clothes
and ornaments etc.
• Usually available in different size, color and shape based on the
manufacturers.
• It is recovered in cases of accident, rape and murder and
kidnapping cases which helps in linking the suspect to scene
of crime.
• Forensic Examination of glitter was performed using
various techniques.
Objective
The main objective of the project is analysis of unusual trace evidence found at the scene of crime. Here we have performed analysis on two types of evidences: Paint and glitter. The forensic of examination of paint and glitter was performed. It involves the physical, microscopic, chemical and instrumental analysis. The automobile manufactures imparts different coatings based on the customers satisfaction which makes each manufacturers unique. Hence in this project we were able to find out the various chemicals or characteristics that were imparted by the manufactures and also the number of layers applied onto the automobile if it is modified. In case of glitter the microscopic analysis of various manufactures helped in revealing the shape and also presence of any contaminants present in it. Also the analysis of the FTIR peaks for the determination functional group present in each of the samples.
Aim
• To perform physical and microscopic examination of paint and
glitter.
• To determine the number of layers in case of microscopic
examination of paint.
• To perform solubility and micro-chemical tests of different
automobile manufacturers.
• To separate the various components of paint using TLC.
• To analyse the FTIR peaks for determining the elemental
composition of paint.
• To determine the specific gravity of glitter.
Methodology
Our project deals with analysis of unusual trace evidence such as paint and glitter. Paint sample specifically automobile paint sample was obtained from various vehicles of different vehicles of different automobile companies. Physical, microscopic and instrumental examination was done on individual paint chip.
Glitter was obtained from different manufacturer such as Vozwa, Tenderberry and Midas. The physical, microscopic and instrumental analysis of each sample was performed.
Physical examination
Paint chips
The physical characteristics of paint chips were examined, such as color, texture, number of layers etc. manually.
Solubility test: The solubility of individual paint sample was tested with various solvents to check the following reaction and solubility.
Micro-chemical test: Test which is used to determine the composition of the paint.
The following solvents were used chloroform, ethyl acetate, methanol, xylene, HCl, H2SO4, HNO3, Methylene Chloride, and Dimethyl Formamide.
Glitter: The physical characteristics of glitter were examined, such as color, texture, shape of particles and edge characteristics etc. were examined manually using hand lens.
Specific density: The specific density of each glitter sample was done using Acetone and a floatation test was conducted to check the specific density.
Microscopic examination
Paint chips and glitter particles: The microscopic examination of paint chips and glitter particles was performed using stereo-microscope. The number of layers and presence of wear and tear marks such as abrasion marks on the surface of the sample were examined.
The number of layers gives idea about the making of each sample and also links it to the manufacturer.
The wear and tear marks gives idea about the age of sample and how it has been used in past.
Instrumental examination
Thin layer chromatography: This technique was used to separate the individual components of paint chips based upon their interaction with the stationary phase and mobile phase. The Rf value of individual samples soluble in appropriate solvent was calculated.
Fourier transforms infrared
It is an instrumental technique based upon the interaction of the molecules of the sample with the electromagnetic waves in the Infrared region.
It usually works by absorption of energy emitted by molecular vibrations, thus giving results in the form of absorption peaks where the intensity of individual peak is studied.
The results show in Tables 1-4. Physical and microscopic examination (Sample no: 1 to 10).
| Model | Color | Physical examination | Year of manufacturing | Microscopic examination | ||
|---|---|---|---|---|---|---|
| Top coat | Base coat | Shape | Texture | Number of layers | ||
| Manu 800 | Silver | Yellowish brown | Irregular (flakes) | Smooth | 1991 | 3 |
| Indica | Silver | Dull white | Irregular | Smooth and shiny | 2008 | 3 |
| Swift dzire | Pearl white | Greyish siter | Irregular | Smooth | 2008 | 3 |
| Creta | Pure white | Grey | Irregular | Smooth | 2014 | 3 |
| Etios | Yellowish | Creamy white | Flakes | Smooth | 2014 | 2 |
| Tata Mahindra | Shiny green | Rusted brown | Regular | Rough due to rust | 1998 | 2 |
| Maruti 800 | Shiny white | Grey | Irregular | Smooth and shiny | 1983 | 3 |
| Tata Safari | White | Brown | Irregular | Smooth | 2012 | 3 |
| Lancer | White | Red | Irregular | Smooth | 2007 | 2 |
| Maruti Suzuki 800 | White | Brown | Irregular (rusted) | Rough | 1983 | 3 |
Table 1. Physical and microscopic examination (sample no: 1 to 10).
| Model | Color | Shape | Texture | Year of manufacturing | Number of layers | |
|---|---|---|---|---|---|---|
| Topcoat | Basecoat | |||||
| Pulsar 180 | Black | Dull black | Irregular (flakes) | Smooth | 2001 | 2 |
| Maruti 800 | Shiny white | Grey | Irregular | Smooth and shiny | 1983 | 2 |
| Mahindra LX | White | Sliver | Regular | Rough | 2003 | 2 |
| Bullet 350 cc | Shiny black | Yellowish brown | Irregular | Smooth | 1974 | 2 |
| Mahindra scorpio | Facelift creamy white | Grey | Irregular | Smooth | 2017 | 2 |
| Mahindra scorpio | Black | Brown | Irregular | Smooth | 2002 | 2 |
| Bullet 250 cc | Red | Creamy brown | Irregular | Matte | 1960 | 3 |
| Indigo Pal | White | Grey | Irregular (rusted) | Rough | 3 | |
| Maruti 800-1985 | Red | Creamy | Regular sharp edges | Smooth | 1985 | 3 |
| Force tempo travellar | White | Brown brown | Irregular (rusted) | Smooth | 1999 | 3 |
Table 2. Physical and microscopic examination sample no: 10 to 20.
| Model | Color | Shape | Texture | Year of manufacturing | Number of layers | |
|---|---|---|---|---|---|---|
| Topcoat | Basecoat | |||||
| GLS Pajero | Maroon Shiny | Brown rusted | Regular and sharp | Rough | 2000 | 2 |
| Toyota qualis | White | Grey | Irregular (rusted) | Smooth | 2001 | 3 |
| Chevrolet FA | Shiny green | Shiny green | Irregular flakes | Smooth | 1941 | 2 |
| Bolero | Creamy white | Black | Irregular flakes | Rough | 2011 | 2 |
| Innova | White | Grey | Irregular | Rough | 2008 | 3 |
| Indigo marina | Shiny silver and black | Dull black | Powdered lustrous | Smooth | 2008 | 2 |
| Mahindra Bolero | Crystal white | Yellowish grey | Irregular | Smooth | 2000 | 3 |
| Fiad | White | Grey | Irregular (rusted) | Rough | 1957 | 3 |
| Scorpio turbo 2.6 | Silver | Powdered | Smooth | 2002 | 2 | |
| Toyota Qualis | Black | Brown | Regular (flakes) | Rough | 2001 | 3 |
Table 3. Physical and microscopic examination sample no: 20 to 30.
| S. no. | Model | Physical examination | Microscopic examination |
|---|---|---|---|
| 1 | Maruti 800 DX |  |
 |
| 2 | Indica |  |
 |
| 3 | Swift dzire |  |
 |
| 4 | Creta |  |
 |
| 5 | Etios |  |
 |
| 6 | Tata mahindra |  |
 |
| 7 | Maruti 800 |  |
 |
| 8 | Tata safari strome |  |
 |
| 9 | lancer |  |
 |
| 10 | Maruti suzuki 800 |  |
 |
| 11 | Maruti suzuki 800 |  |
 |
| 12 | Maruti 800 |  |
 |
| 13 | Mahindra LX |  |
 |
| 14 | Bullet 350 cc |  |
 |
| 15 | Mahindra scorpio facelift |  |
 |
| 16 | Mahindra scorpio |  |
 |
| 17 | Bullet 250 cc |  |
 |
| 18 | Indigo pal |  |
 |
| 19 | Maruti 800(1985) |  |
 |
| 20 | Force tempo traveller |  |
 |
| 21 | GLS Pajero |  |
 |
| 22 | Toyota gualis |  |
 |
| 23 | Chevrolet FA |  |
 |
| 24 | Bolreo |  |
 |
| 25 | Innova |  |
 |
| 26 | Indigo marina |  |
 |
| 27 | Mahindra bolero |  |
 |
| 28 | FIAD |  |
 |
| 29 | Scorpio turbo 2.6 |  |
 |
| 30 | Toyota qualis |  |
 |
Table 4. Detail explanation about physical examination and microscopic examination.
Physical examination: The physical feature of the paint was observed which includes the color, size, texture and shape and year of manufacturing. It helps in documentation, for describing the general condition as well as the easy evalution of the sample.
Microscopic examination:
• It is done by using stereomicroscope for determining the number of layers, surface markings, formation of rust, modification applied onto the coating by applying more number of coatings to change the topcoat color
• Physical matching: The matching of the sample from the broken pieces obtained along their edges with the control samples.
• Layers matching: It consists of several layers such as topcoat, basecoat and clearcoat. Whether it matches with the suspected sample obtained from the crime scene to those of the control sample based on the Law of comparison.
• Sequences of layer determination
• Repainted vehicles have difference in layers when the matching of layers is performed.
• Surface markings: Scratch marks, texture, any material adhered onto the surface.
• Different automobile manufactures have incorporated different color combination or coating process depending upon the cutomers satisfaction or needs in the market which makes them unique. Hence the layers observed are different for different manufacturers (Tables 5-8).
| S. no | Model | Acetone | Chloroform | Characteristics observed | |
|---|---|---|---|---|---|
| Acetone | Chloroform | ||||
| 1 | Marruti 800 DX | Not soluble | Soluble | No change | Curling along the edges |
| 2 | Indica | Not soluble | Soluble | No change | Curling along the Softening |
| 3 | Swift desire (pearl white) | Not soluble | Soluble | No change | Curling |
| 4 | Creta (pure white) | Not soluble | Soluble | No change | Curling along the edges, softening with cracks |
| 5 | Etios | Not soluble | Soluble | No change | Colour fading and softening |
| 6 | Tata mahindra | Soluble | Soluble | Colour fading | Colour fading |
| 7 | Maruti 800 | Not soluble | Soluble completely | No change | Forms cracks and effervescence |
| 8 | Tata safari strom | Soluble completely | Not soluble | Formation of cracks | |
| 9 | Lancer | Not soluble | Not soluble | No change | No change |
| 10 | Maruti suzuki 800 | Soluble | Soluble | Colour fading | No change |
| 11 | Pulsar 180 | Soluble | Soluble | Softening | Colour fading |
| 12 | Maruti 800 | Soluble | Soluble | Curling along the edges, Colour faded to grey | No change |
| 13 | Mahindra LX | Soluble completely | Soluble | Separated into two layers | |
| 14 | Bulet 350 cc | Soluble | Not soluble | No change | Curling along the edges and softening |
| 15 | Mahindra scorpio facelift | Soluble | Not soluble | No change | Separated into two layers |
| 16 | Mahindra scorpio | Soluble | Not soluble | Softening | No change |
| 17 | Bulet 250 | Soluble | Soluble | No change | Efferevescence, colour fading |
| 18 | Indigo pal | Soluble | Soluble | No change | Colour fading |
| 19 | Maruti 800 (1985) | Soluble | Soluble | No change | Colour fading and formation of cracks |
| 20 | Fce tempo traveller | Soluble | Not soluble | No change | No change |
Table 5. Solubility tests acetone and chloroform sample no: 1 to 20.
| S. no | Model | Acetone | Choloroform | Characteristics observed | |
|---|---|---|---|---|---|
| Acetone | Chloroform | ||||
| 21 | GLS Pajero | Not soluble | Soluble | No change | Color fading |
| 22 | Toyota Quals | Not soluble | Sobble | No change | Softening |
| 23 | Chevrolet FA | Not soluble | Soluble | No change | Curing |
| 24 | Bolereo | Not soluble | Soluble | No change | Curing softening |
| 25 | Innova | Not soluble | Soluble | No change | Curling softening |
| 26 | Indigo marina | Not soluble | Not soluble | No change | No change |
| 27 | Mahindra Bolero | Not sobble | Soluble | No change | Curling |
| 28 | FLAD | Soluble | Not soluble | Separate layers | No change |
| 29 | Scorpio turbo 2.6 | Not solibble | Soluble | No change | Curing |
| 30 | Toyota Quals | Not soluble | Not soluble | No change | No change |
Table 6. Solubility test acetone and chloroform sample no: 21 to 30.
| S. no | Model | Cone HCl | Conc H2SO4 | Conc HNO3 |
|---|---|---|---|---|
| 1 ) | Maruti 800 | Color change-yellow | Color change-light red | No change |
| 2) | Indica | No change | Color change and hissing | No change |
| 3) | Swift dire | Effervescence hissing sound | Slight color change | Color change-pale yellow |
| 4) | Creata | No change | Color change-light brown | Color change-pale yellow |
| 5) | Etios | No change | Slight color change | No change |
| 6) | Tata mahindra | Color change-deep yellow | Color faded | Color change-pale brown |
| 7) | Maruti 800 | No change | Color change-pale brown | No change |
| 8) | Tata safari storm | Color change-pale yellow | Color change-light brown | No change |
| 9) | Lancer | Color change-grey | Color change-light brown | Color change-pale yellow |
| 10) | Maruti suzuki 800 | Color change-pale yellow | No change | No change softening |
| 11) | Pulsar 180 | No change | No change | No change |
| 12) | Maruti 800 | No change | Color change-pale yellow | No change |
| 13) | Mahindra LX | Color change-pale yellow | Color change-pale Brown | No change |
| 14) | Bullet 350 cc | No change | Color change-pale brown | No change |
| 15) | Mahindra scorpio facelift | No change | Color change-pale yellow | No change |
| 16) | Mahindra scorpio | Color change-deep yellow | Color change-pale brown | Color change-pale brown |
| 17) | Bullet 250 cc | Color change-deep yellow | Color change-pale red | Color change-pale red |
| 18) | Indigo pal | Color change-yellow | No change | No change |
| 19) | Maruti 800 (1985 ) | Color change-pale yellow | Color change-deep red | No change |
| 20) | Force Tempo traveller | Color change-deep yellow | Color change-pale yellow | No change |
Table 7. Microchemical test conc HCl, conc H2SO4, conc HNO3 sample No: 1 to 20.
| S. no | Model | Conc HCl | Conc H2SO4 | Conc HNO3 |
|---|---|---|---|---|
| 21) | Gls pajero | Color change-deep yellow | Color change-purple | Color change-red |
| 22) | Toyota qualis | Color change-pale yellow | Color change-pale brown | No change |
| 23) | Chevrolet FA | Color change-yellow | Color change-pale brown | No change |
| 24) | Bolereo | No change | Color change-pale brown | No change |
| 25) | Immova ( 2008 ) | No change | No change | Color change-pale yellow |
| 26) | Indigo menina | No change | Color change-deep brown | Color change-pale yellow |
| 27) | Mahindra bolereo | Color change-deep yellow | Color change-pale brown | No change |
| 28) | FIAD | No change | Color change-pale brown | No change |
| 29) | Scorpio turbo 2.6 | No change | No change | No change |
| 30) | Toyota quals | Color change-pale yellow | Color change-dark green | No change |
Table 8. Microchemical and solubility test conc HCl, conc H2SO4, conc HNO3 sample No: 21 to 30.
Micro chemical tests
• Diphenyl amine reagent: 0.3 gm of diphenylamine in 20 ml H2SO4 and glacial acetic acid (Table 9).
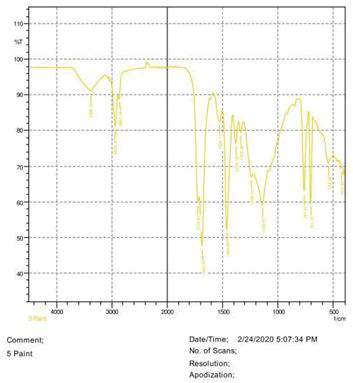
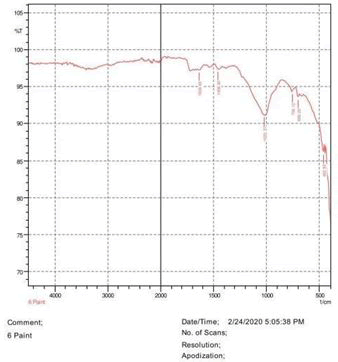
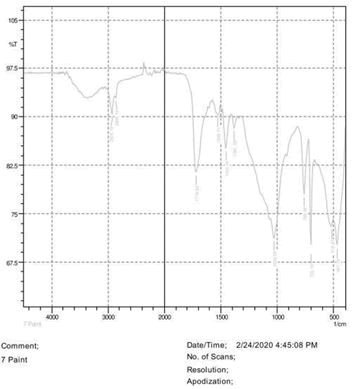
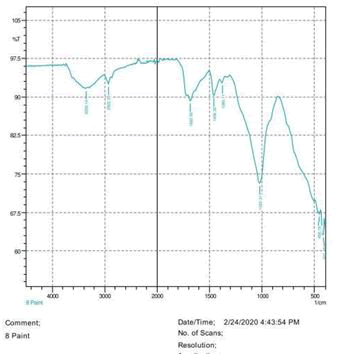
• Le Rosen reagent: 10 drops of 37% formaldehyde added drops by drop to 10 ml of Sulphuric acid (Figures 1-8).
| Reagents | Results |
|---|---|
| Conc NaOH | No change |
| Le Rosen reagent | No change |
| Diphenylamine | No change |
| Benzene | No change |
| Methanol | No change |
| Xylene | No change |
| Ethyl acetate | No change |
| Ammonium sulphide | No change |
Table 9. Reagents and results.
Observation
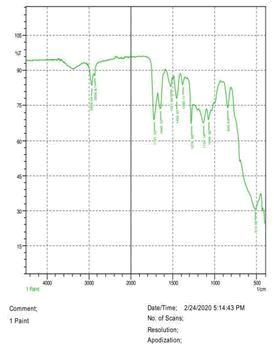
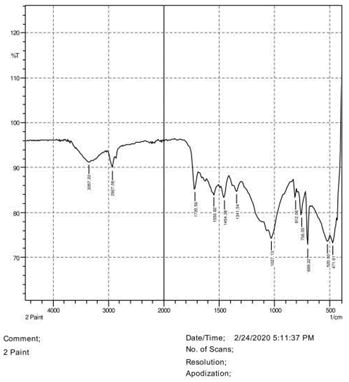
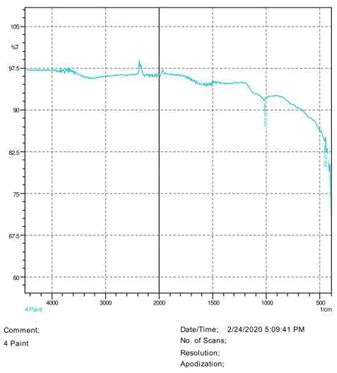
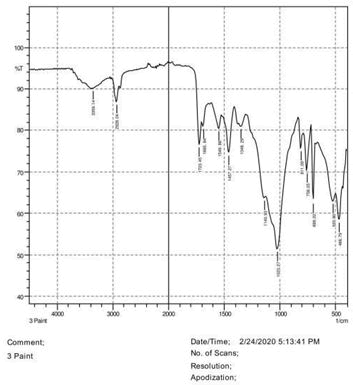
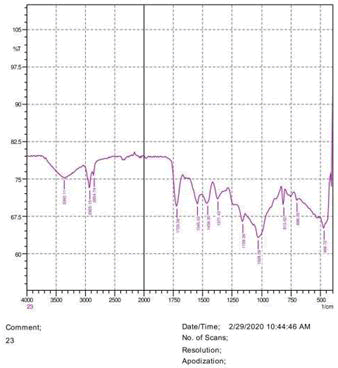
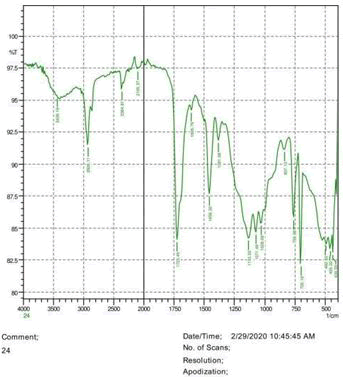
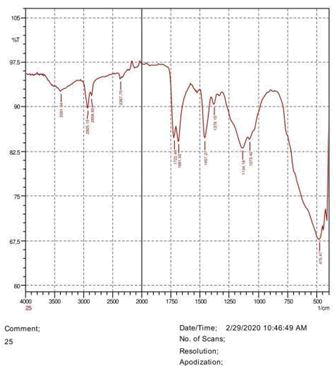
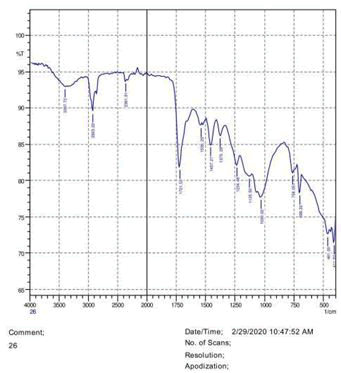
The observations of acetone and chloroform show in Figures 1-4.
Observation
The observations of Conc HCL, conc H2SO4, conc HNO3 show in Figures 5 and 6.
Observation
The observations of Conc. NaOH and ammonium sulphide show in Figures 7 and 8.
Solubility test:
• Acrylic lacquers: Soluble (Chloroform, acetone).
• Nitrocellulose lacquers: Soluble (acetone).
• Nitrocellulose lacquers: Insoluble (Chloroform).
• Enamels: Insoluble(acetone, chloroform).
Microchemical tests
• Paint chips are tested using the chemicals which lead to various chemical reactions that help in identifying the components in the paint such as pigment, binder, additives etc.
• Almost all the samples show reaction such as effervescence, cracks formation, color change, softening etc. with conc H2SO4 and conc HCL.
• Paint chips having carbonates gives off bubbles or hissing sound with strong acids.
• Conc HNO3 showed very less compared to the conc H2SO4 and conc HCL among different manufacturers.
• Ethyl acetate, Ammonium sulphide, conc NaOH, Le Rosen reagent, Diphenyl amine reagent, Benzene, Methanol and xylene gave negative results with all the paint chips (Figure 9).
Retardation factor
Rf=Distance travelled by solute/distance travelled by solvent.
Rf value: 7.8, 8.9, 9.5, 10, 6.5
The paint chips which are soluble in acetone and chloroform were separated using TLC. The components get separated based on the affinity towards stationary phase.
Instrumentation Fourier transform infrared
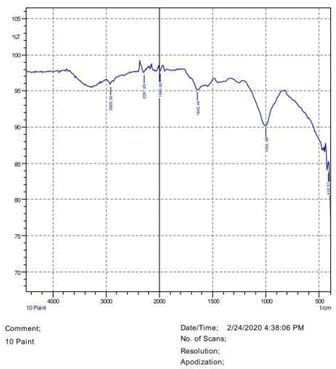
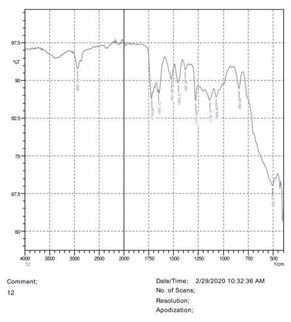
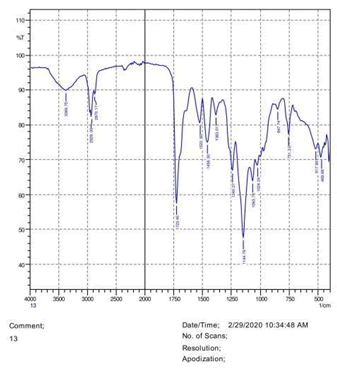
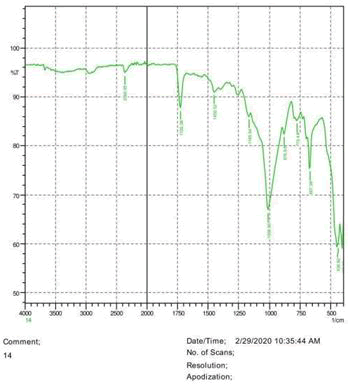
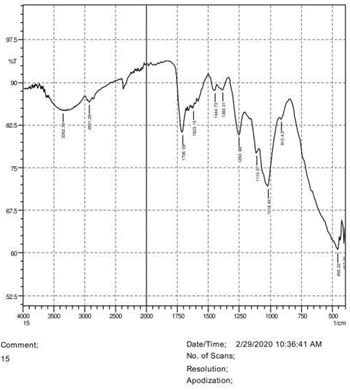
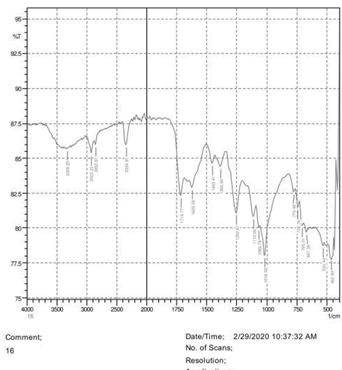
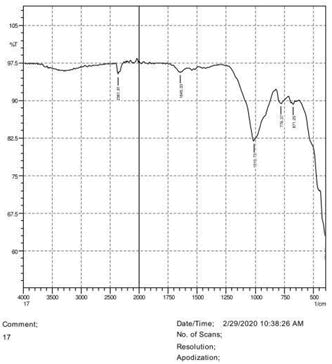
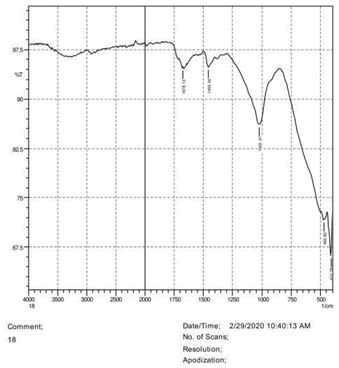
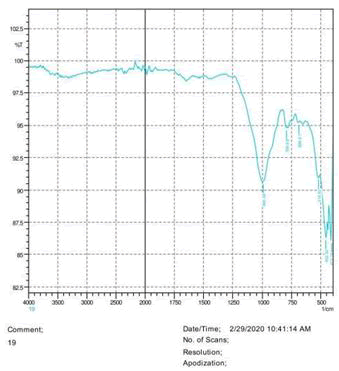
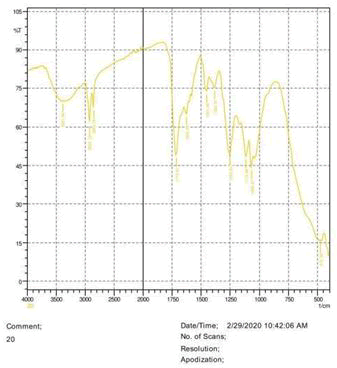
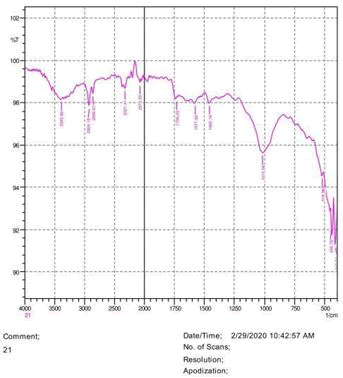
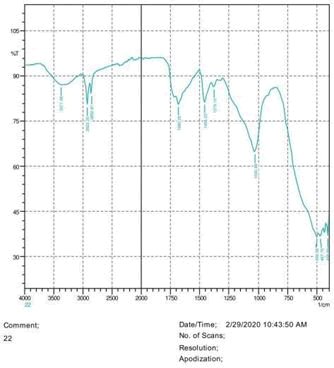
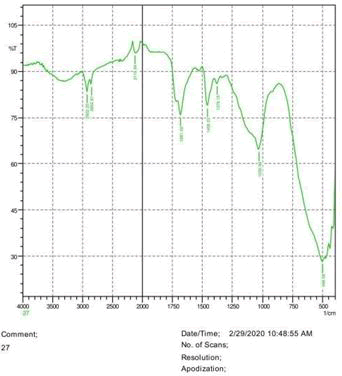
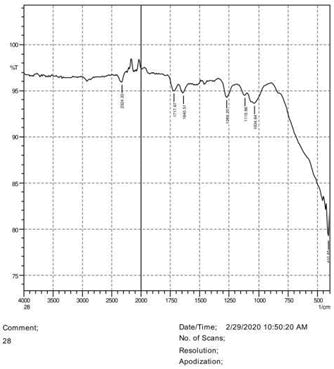
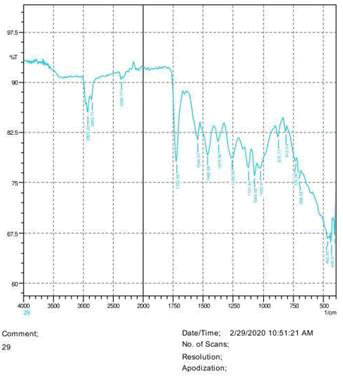
• The FTIR peak examination gives idea about the composition tohfe given sample such as the functional group present and the molecular vibration associated. A graphical representation of absorption peak v\s intensity gives the exact idea about the composition. The automobile paint samples were submitted for FTIR peak examination and the following information was derived:
• Almost 28 samples were given for examination and the absorption peak of every individual sample was obtained.
• Functional groups and their molecular vibration were observed. Alcohol derivatives, ketone, Aldehyde, Esters, Ether, Halogen, Nitro Groups, Amine, Sulfur compounds i.e. sulfoxide; sulfone and sulfonate etc. were found to be present in the automobile paint samples. The composition varies according to the model and the pigment used (Table 10).
| FTIR peak | Absorption (cm-1) | Group | Interpretation |
|---|---|---|---|
 |
1134.18 1452.45 1645.33 1721.53 2856.67 2928.04 |
C-O stretching C-H bending C=C stretching C=O stretching C-H stretching O-H stretching |
Tertiary alcohol Alkane Vinylidene Cyclohexane Cyclopentanone Aldehyde Alcohol |
 |
759.05 812.06 1027.13 1341.54 1454.38 1550.82 1720.56 2927.08 3357.22 |
C-H bending C-H bending S=O stretching C-N stretching C-H bonding N-O stretching C=O stretching C-H stretching N-H stretching |
1,2 disubstituted 1,4 disubstituted Sulfoxide Aromatic amine Methyl group Nitro Compound Dimer, conjugated acid Alkane Aliphatic primary amine |
 |
1015.56 | S=O stretching | Sulfoxide |
 |
811.09 1023.27 1140.93 1348.29 1457.27 1549.86 1685.84 1723.45 2928.04 3359.14 |
C-H bonding N-O stretching N-O stretching O-H bending N-O stretching C=N stretching C=O stretching C=O stretching N-H stretching C-H stretching |
1,4 disubstituted Hydrate Hydrate Intramolecular bond Carboxylic acid Nitro group Imine/oxime Conjugated acid Amine salt Alkyne |
 |
761.91 1139.97 1333.82 1378.18 1459.2 1526.71 1683.91 1719.6 2961.49 2929.97 3339.15 |
C-H bending C-O stretching S=O stretching S=O bending C-H bending N-O stretching C=O stretching C=O stretching C-H stretching C-H stretching N-H stretching |
1,2,3-trisubstituted Secondary alcohol Sulfonamide Sulfate Alkane Nitro group Conjugated ketone Carboxylic acid Alkane Alkane Aliphatic primary amine |
 |
699.22 756.12 1023.27 1456.3 1636.65 |
C=C stretching C-H bending S=O stretching O-H bending C=C stretching |
Alkene Monosubstitute Sulfoxide Dimer Alkene |
 |
700.18 759.98 1028.09 1381.00 1452.45 1526.71 1719.63 2854.74 29324.18 |
C-H bending C-H bending C-O stretching O-H bending C-H bending N-O stretching C-O stretching C-H stretching C-H stretching |
1,2,3,4,5,6, substituted 1,2-disubstitude Vinyl ether Phenol Alkane Nitro group Carboxylic acid Alkane Alkane |
 |
1022.31 1380.11 1456.3 1682.95 2922.25 3359.14 |
C-O stretching O-H bending C-H bending C=O stretching C-H stretching N-H stretching |
Alkyl aryl ether Phenol Alkane Conjugated ketone Alkane Secondary amine |
 |
1003.98 1642.44 1990.6 2287.65 2920.32 |
C=C bending C=C stretching C-H bending N=C=O stretching C-H stretching |
Alkene Alkene Aromatic compound Nitrile Alkane |
 |
840.99 1069.56 1135.15 1276.92 1381.08 1453.41 1521.89 1651.12 1719.6 2931.9 |
C-H bending S=O stretching C-O stretching C-O stretching O-H bending C-H bending N-O bending C=N stretching C=O stretching O-H stretching |
1,2 disubstituted Sulfoxide Secondary alcohol Aromatic ester Phenol Methyl group Nitro compound Oxime Dimer Carboxylic acid |
 |
751.3 847.74 1024.24 1063.78 1144.79 1240.27 1383.01 1456.3 1522.85 1723.45 2870.17 2929 3369.75 |
C-H bending C-H bending S=O stretching C-O stretching S=O stretching C-O stretching O-H bending C-H bending N-O stretching C=O stretching C-H stretching C-H stretching N-H stretching |
1,2 dsiubstituted 1,4 disubstituted Sulfoxide Alkyl aryl ether Sulfone Alkyl aryl ether Phenol Alkane Nitro compound aß-unsaturated ester Alkane Alkane Aliphatic primary amine |
 |
773.48 876.68 1008.8 1165.04 1450.52 1725.38 2380.95 |
C-H bending C-H bending C=C stretching N-O stretching O-H bending C=O stretching C=C stretching |
1,2-disubstituted 1,2,3-trisubstituted Alkene Nitro compound Phenol Carboxylic acid Alkyne |
 |
910.43 1018.45 1110.07 1250.88 1383.01 1444.73 1623.15 1706.09 2921.29 3352.39 |
N-O stretching O-O stretching C-O stretching C-F stretching C-N stretching O-H stretching C=C stretching C=O stretching O-H stretching O-H stretching |
Nitro compound Nitro compound Tertiary alcohol Fluoro compound Aromatic amine Carboxylic acid Conjugated alkene Carboxylic acid Carboxylic acid Carboxylic acid |
 |
705.01 739.76 773.48 1016.52 1063.78 1112 1252.81 1385.9 1453.41 1620.26 1714.77 2334.91 2852.81 2923.22 3329.25 |
C-H bending C-H bending C-H bending C=C bending S=O stretching C-O stretching C-O stretching O-H bending C-H bending C=C stretching C=O stretching O=C=O stretching C-H stretching C-H stretching N-H stretching |
Benzene derivative 1,2-disubustituted 1,2-disubstituted Alkene Sulfoxide Secondary alcohol Alkyl aryl ether Phenol Alkane aß-unsaturated ketone Carboxylic acid Carboxylic acid Alkane Alkane Aliphatic primary amine |
 |
671.25 776.37 1010.73 1645.33 2361.91 |
C-Br stretching C-Cl stretching C-F stretching C=N stretching C=N stretching |
Halo compound Halo compound Fluoro compound Imine Nitrile |
 |
1022.31 1455.34 1678.13 |
N-O stretching C-F stretching C=O stretching |
Nitro group Fluoro compound Carboxylic acid |
 |
688.61 789.88 990.48 |
C-Br stretching C-Cl stretching C-F stretching |
Halo compound Halo compound Halo compound |
 |
1066.67 1112.96 1252.81 1383.01 1451.48 1625.08 1713.81 2853.78 2923.22 3391.94 |
S=O stretching C-O stretching C-N stretching O-H bending C-H bending C=C stretching C=O stretching C-H stretching C=H stretching C=C stretching |
Sulfoxide Secondary alcohol Amine Phenol Alkane aß unsaturated ketone Carboxylic acid Alkane Alkene alkyne |
 |
1015.56 1460.16 1577.82 1735.03 2073.55 2321.41 2856.67 2924.18 3393.86 |
C-O stretching C-H bending C=C stretching C=O stretching N=C=S stretching O=C=O stretching C-H stretching C-H stretching N-H stretching |
Vinyl ether Alkane Cyclic alkane Aldehyde Isothiocynate Carbondioxide Alkane Alkane Aliphatic primary amine |
 |
1030.99 1379.15 1458.23 1680.05 2852.81 2922.25 3371.68 |
S=O stretching O-H bending C-H bending C=O stretching N-H stretching N-H stretching N-H stretching |
Sulfoxide Phenol Alkane Secondary amide Amine salt Amine salt Aliphatic primary amine |
 |
813.02 1028.09 1159.26 1371.43 1459.2 1545.03 1720.56 2854.74 2925.15 3360.11 |
C-H bending S=O stretching S=O stretching S=O stretching C-H bending N-O stretching C=O stretching C-H stretching C-H stretching N-H stretching |
1,4-disubstituted Sulfoxide Sulfone Sulfonamide Alkane Nitro compound aß unsaturated ester Alkane Alkane Aliphatic primary amine |
 |
759.98 837.13 1028.09 1071.49 1133.22 1381.08 1451.3 1605.79 1723.45 2105.37 2364.81 2926.11 |
C-H bending C-H bending S=O stretching C-O stretching C-O stretching C-N stretching C-H bending N-O stretching C=O stretching C=C stretching O=C=O stretching N-H stretching |
Monosubstitute 1,4-disubstituted Sulfoxide Secondary alcohol Aliphatic ether Aromatic amine Alkane Nitro compound Aliphatic ketone Alkyne Carbondioxide Amine salt |
 |
1073.42 1134.18 1379.15 1457.27 1681.02 1722.49 2367.7 2858.6 2925.15 3391.94 |
C-O stretching C-O stretching O-H bending C-H bending C=O stretching C=O stretching N=C=O stretching C-H stretching C-H stretching N-H stretching |
Primary alcohol Aliphatic ether Phenol Alkane Primary amide Aliphatic ketone Isocyanate Alkane Alkane Aliphatic primary amine |
 |
1030.02 1125.5 1234.48 1375.29 1457.27 1539.25 1721.53 2361.91 2923.22 3397.72 |
S=O stretching C-O stretching C-N stretching C-H bending O-H bending N-O stretching C=O stretching O=C=O stretching C-H stretching N-H stretching |
Sulfoxide Aliphatic ether Amine Alkane Carboxylic acid Nitro compound Aliphatic ketone CO2 Alkane Aliphatic primary amine |
 |
1030.99 1379.15 1458.23 1681.02 2119.84 2852.81 2922.25 |
S=O stretching O-H bending C-H bending C=N stretching N=C=S stretching C-H stretching C-H stretching |
Sulfoxide Alcohol Alkane Imine Isothiocynate Alkane Alkane |
 |
1034.84 1115.86 1269.2 1640.51 1717.67 2324.3 |
S=O stretching C-O stretching C-O stretching C=C stretching C=O stretching O=C=O streching |
Sulfoxide Aliphatic ether Alkyl aryl ether Conjugated alkene Aliphatic ketone Carbondioxide |
 |
812.06 875.71 1022.31 1069.56 1121.64 1258.59 1373.36 1462.09 1544.07 1720.56 2365.77 2855.71 2923.22 |
C-H bending C-H bending C=C bending S=O stretching C-O stretching C-O stretching S=O stretching C-H bending N-O stretching C=O stretching N=C=O stretching C-H stretching N-H stretching |
1,2,4-trisubstituted 1,2,4-trisubstituted Alkene Sulfoxide Secondary alcohol Alkyl aryl ether Sulfonate Alkane Nitro compound Carboxylic acid Isocynate Alkane Amine salt |
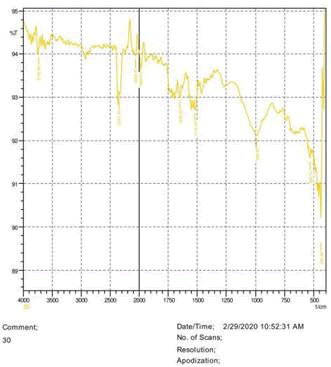 |
988.55 1518.03 1648.23 1989.64 2064.87 2347.45 3743.96 |
C=C bending N-O stretching C=C stretching C-H bending N=C=S stretching O=C=O stretching O-H stretching |
Alkene Nitro compound Alkene Aromatic compound Isothiocyante CO2 Alcohol |
Table 10. The FTIR peak examination gives idea about the composition of the given sample.
Glitter
Types of glitter
• Holographic glitter: It is a type of glitter that reflects the incident light into different colors.
• Metallic glitter: It is not made of glitter but it denotes the property to shine like a metal.
• Irridescent glitter: It is a type which is available in different shades.
Particle size
• Large size: 0.5 mm-0.8 mm
• Normal size: Less than 0.2 mm (most commonly used).
• Physical analysis: Different manufactures have different colors.
Shape and size
• Tenderberry and Vozwa: Hexagonal, circular, polygonal, pentagonal
• Middas: polygonal
Specific gravity: The glitter particles sink in acetone which states
During the microscopic examination of Middas manufacutrer there was contaminants such as sand was present in the samples. The adulterants were added to enhance the shinning property of the glitter or to increase the quantity of glitter particles (Tables 11 and 12).
| Manufacturer | Color | Texture | Shape | Specific gravity | ||
|---|---|---|---|---|---|---|
| VOZWA | 1) | V1 | Red shimmer | Smooth | Hexagonal | Sink |
| 2) | V2 | Red matte | Smooth | Circular/Spherical | Sink | |
| 3) | V3 | Blue matte | Smooth | Spherical | Sink | |
| 4) | V4 | Silver | Smooth | Hexagonal | Sink | |
| 5) | V5 | Orange matte | Smooth | Polygonal | Sink | |
| 6) | V6 | Golden shimmer | Smooth | Hexagonal | Sink | |
| 7) | V7 | Golden matte | Smooth | Pentagonal | Sink | |
| 8) | V8 | Dark green | Smooth | Hexagonal | Sink | |
| 9) | V9 | Orange | Smooth | Hexagonal | Sink | |
| 10) | V10 | Silver matte | Smooth | Polygonal | Sink | |
| 11) | V11 | Pink | Smooth | Hexagonal | Sink | |
| 12) | V12 | Pink matte | Smooth | Spherical | Sink | |
| TENDERBERY | 13) | T1 | Baby pink | Rough | Spherical | Sink |
| 14) | T2 | Magenta pink | Rough | Polygonal | Sink | |
| 15) | T3 | Blue matte | Rough | Polygonal | Sink | |
| 16) | T4 | Dark brown | Rough | Polygonal | Sink | |
| 17) | T5 | Multi-color | Rough | Polygonal | Sink | |
| 18) | T6 | Green | Rough | Hexagonal | Sink | |
| 19) | T7 | Golden | Rough | Polygonal | Sink | |
| 20) | T8 | Purple | Rough | Polygonal | Sink | |
| 21) | T9 | Black | Rough | Polygonal | Sink | |
| 22) | T10 | Brown | Rough | Hexagonal | Sink | |
| 23) | T11 | Red shimmer | Rough | Polygonal | Sink | |
| 24) | T12 | Rose orange | Rough | Polygonal | Sink | |
| MIDDAS | 25) | Ml | Golden | Smooth | Polygonal | Sink |
| 26) | M2 | Green | Smooth | Polygonal | Sink | |
| 27) | M3 | Red | Smooth | Polygonal | Sink | |
| 28) | M4 | Pink | Smooth | Polygonal | Sink | |
| 29) | MS | Silver | Smooth | Polygonal | Sink |
Table 11. Graphical representation of gliter.
| Tenderbery | Shape | Colour | Texture | Image | Vozwa | Shape | Colour | Texture | Image | Middas | Shape | Colour | Texture | Image |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | Spherical | Baby pink | Rough |  |
V1 | Hexagonal | Red shimmer | Smooth | 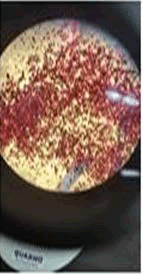 |
M1 | Polygonal | Golden | Smooth | 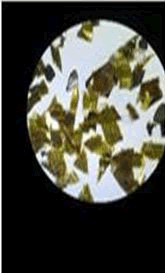 |
| T2 | Polygonal | Magnet A pink | Rough |  |
V2 | Circular/Spherical | Red matte | Smooth |  |
M2 | Polygonal | Green | Smooth | 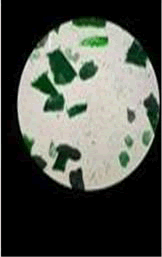 |
| T3 | Polygonal | Blue matte | Rough |  |
V3 | Spherical | Blue matte | Smooth |  |
M3 | Polygonal | Red | Smooth | 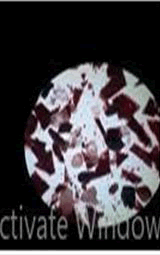 |
| T4 | Polygonal | Dark brown | Rough | 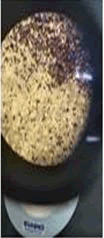 |
V4 | Hexagonal | Silver | Smooth | 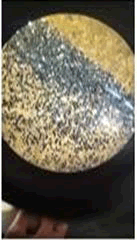 |
M4 | Polygonal | Pink | Smooth |  |
| T5 | Hexagonal | Multi-colour | Rough | 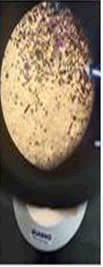 |
V5 | Polygonal | Orange matte | Smooth |  |
M5 | Polygonal | Silver | Smooth | 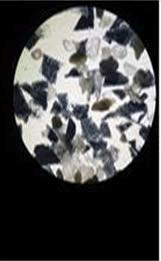 |
| T6 | Hexagonal | Green | Rough |  |
V6 | Hexagonal | Golden shimmer | Smooth | 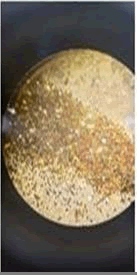 |
M6 | ||||
| T7 | Hexagonal | Golden | Rough |  |
V7 | Pentagonal | Golden matte | Smooth |  |
M7 | ||||
| T8 | Hexagonal | Purple | Rough | 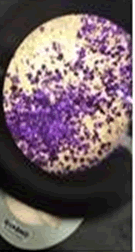 |
V8 | Hexagonal | Dark green | Smooth |  |
M8 | ||||
| T9 | Hexagonal | Black | Rough |  |
V9 | Hexagonal | Orange | Smooth |  |
M9 | ||||
| T10 | Hexagonal | Brown | Rough |  |
V10 | Polygonal | Silver matte | Smooth | 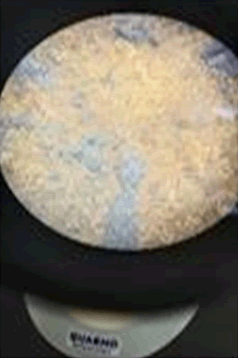 |
M10 | ||||
| T11 | Polygonal | Red shimmer | Rough | 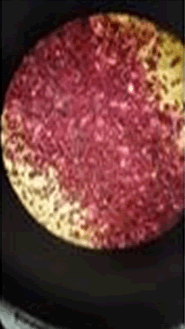 |
V11 | Hexagonal | Pink | Smooth |  |
M11 | ||||
| T12 | Polygonal | Rose orange | Rough |  |
V12 | Spherical | Pink matte | Smooth |  |
M12 |
Table 12. Explanation about glitters.
Paint is the coating substance that is implemented on metallic and non-metallic surfaces for decorative or protection purposes. It is composed of four components pigment, binder, liquid and additives. Application method can depend on the types of paint it can include spray, brush, and electrostatic spraying. Paint is mainly of two types, based on physical state and based on function. Primer, first coat applied directly on a steel surface and also provides surface protection from corrosion. Base coat is applied after the primer and helps in providing a visual color effects. Clear coat is placed on the top of the base coat. It does not contain any color and provide protection to the paint and to make it look shiny. It consists of UV inhibitor's as paint gets damaged because of the sun's UV rays. Paint chips are found very useful in the forensic field. Paint chips are used in cases involving hit and run cases, burglary case, sexual assault cases, homicide and kidnapping. These evidences are collected, preserved and packaged. In hit and run cases, it is removed using sharp object such as knife, blade and layers of paint is scrapped from the vehicle. These collected paint chips are analyzed. Firstly, physical examination is done on the evidence, which involves color, size, texture of evidence and then state of evidence. After physical examination, the evidence is analyzed under the stereomicroscope for determining the number of layers, surface marking and distribution of pigments using stereomicroscope. Various chemical test are also performed on these evidences; microchemical test, solubility test and TLC.
Inorganic solvent which includes conc. HCl, conc. H2SO4, and conc. HNO3 was used for microchemical tests. Various types of reactions was observed such as color change, bubbling, effervescence, softening, curling and formation of cracks was observed after the addition of reagents. Thin layer chromatography was performed and calculated the Rf factors based on the chromatogram which was obtained after visualization. The elemental analysis of various paint chips was done using Fourier Transform Infrared (FTIR). It helps in determine the molecular functional groups in the paint chips based on the peaks obtained.
Glitters are sparkling material which shines by reflecting bright and shimmery light at different angles. It is entirely man-made. It is made up of many of tiny pieces of different material such as aluminum foil, titanium dioxide, copolymer plastic and iron oxides. In prehistoric time glitter was made from stones such as malachite and mica and also from some insects and glass. Glitter is used to make cosmetic products, craft, projects, clothes, decorative items etc. Glitter is of main three types; holographic glitter, metallic glitter and iridescent glitter. It is classified on the basis of shape, size and transparency. Glitter can also be important in the forensic as trace evidence eg. Sexual assault case, kidnapping, hit and run cases, homicide etc. Currently glitter is used in many products, cosmetic, designer clothes or bags etc. It is hardly visible, easily transferable, can tolerate extreme condition, maximum retention time, least degradation. We can locate glitter by using flashlights and can be collected using post-it note. In zip-lock bags packaging is done. Physical examination, microscopic examination and instrumental analysis are conducted. Color of glitter, shape, size, thickness, specific gravity is examined. Techniques used for instrumental analysis ATR-FTIR, dispersive Raman micro spectroscopy with confocal imaging, scanning electron microscopy.
Color is physically examined. Using stereomicroscope shape and size of glitter was examined. Glitters are manufactured in different shapes such as hexagonal, square, rectangle, diamond etc. Specific gravity can be measured using ethanol, suspend the glitter in acetone and then observe whether it sinks or floats. Here most of the glitter particles sink which states that the specific gravity of glitter is more than that of the solvent used.
It gives us great pleasure to complete and submit this thesis entitled. Analysis of unusual trace evidence-paint and glitter within a stipulated time. We hope that this work will be of immense value.
We would like to express our sincere gratitude to our supervisor Kiran Yadav under whose precious guidance, support and encouragement to work on this dissertation topic more effectively and smoothly. She helped us in all the ways from the very beginning and always ready to help us at any cost. We are indebted to thank her for her valuable support throughout the project.
We would also like to thank all the people who gave us all the possible ideas, suggestions and guidance for completing this project on time.
We would like to express our gratitude towards HOS Neeta Raj Sharma, HOD Jaskaran Singh and HOL Chirag Chopra for the allocation of lab and for their support for completing the project.
We would also express our heartfelt gratitude to our father, mother. They were always with us and give their constant support and encouraging us during completing our work.
At last but not the least we wish to express our humblest thank to Lab attendants Shamsher Singh, Omkar Chand and Manoj kumar who helped us by providing essential equipment and also helped in providing results for instrumentations which was needed to complete our dissertation work.
I hereby declare that the project report entitled analysis of Unusal Trace Evidence-Paint and Glitter” submitted to the School Bioengineering and Biosciences, Lovely Professional University for the award of degree of Bachelor of science (B. Sc.) is a record of original and independent research work done by us under the supervision and guidance of Kiran Yadav, Assistant professor (Forensic Science), School of Bioengineering and Bio- Sciences, Lovely Professional University. This work has not been submitted for any other degree or professional qualification except as specified.
[Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Journal of Forensic Research received 2328 citations as per Google Scholar report