Research Article - (2023) Volume 13, Issue 4
Received: 02-Aug-2023, Manuscript No. jbbs-23-110476;
Editor assigned: 03-Aug-2023, Pre QC No. P-110476;
Reviewed: 17-Aug-2023, QC No. Q-110476;
Revised: 22-Aug-2023, Manuscript No. R-110476;
Published:
29-Aug-2023
, DOI: 10.37421/2155-9538.2023.13.366
Citation: Rani, Rakhshanda and Muhammad Irfan. “Association
of MTHFR C677T with Obesity in Human Female Population.” J Bioengineer &
Biomedical Sci 13 (2023): 366.
Copyright: © 2023 Rani R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The hypothalamo-pituitary-thyroid axis is responsible for the maintenance of the metabolic processes in the human body. The hypothalamus releases Thyroid Releasing Hormone (TRH) which stimulates the pituitary gland to release Thyroid Stimulating Hormone (TSH) which in turn leads to stimulation of the thyroid gland and release of Thyroxine (T4) and its active derivative Triiodothyroxine (T3). Malfunctioning of any component at any level of the hierarchy of the axis may lead to disorders of metabolism including obesity. The Thyrotropin Releasing Hormone (TRH) is a hypothalamic peptide hormone that possesses a broad spectrum of effects mainly determined by its stimulatory effects on energy metabolism together with iodine containing hormones of the thyroid. The objective of this study is to determine association of a specific Mthfr single nucleotide polymorphism with obesity (BMI) in human at early age. This research included the obese and normal females visiting the hospitals of Islamabad and Rawalpindi. The data about socio-demographic, physical health and lifestyle aspects was collected in the form of a predefined questionnaire Blood samples were collected. Using the traditional phenol-chloroform procedure, DNA was extracted from blood samples and kept at -20℃. The particular primers were created and enhanced for the gene. A particular restriction enzyme was used to digest the PCR-amplified products in order to identify polymorphisms. The digested product was electrophoresed with ethidium bromide staining on agarose gel, and the results were then seen using UV trans illumination. The DNA fragments of the wild-type and mutant varieties were obtained on the gel. The allele frequency of the C to T polymorphism was determined by counting alleles through electrophoresis gel analysis. Chi-square analysis was used to determine the Hardy- Weinberg equilibrium of the alleles in the population. By adjusting the effects of confounding factors such age and socioeconomic characteristics, logistic regression analysis was used to establish the correlation between the polymorphism and BMI. Statistical significance was set at a p value of<0.05. MTHFR C677T was not linked to obesity in women according to our research. The present study has some limitations, including the fact that the subjects' levels of homocysteine and folate were not assessed. A follow-up research is therefore required to assess the folate and homocysteine levels in the obese patients in addition to the MTHFR C677T.
Obesity • SNP • MTHFR • Folate • Homocysteine • Phenotype • Genotype
Methylenetetrahydrofolate Reductase (MTHFR) catalyzes the reduction of 5, 10-Methylenetetrahydrofolate to 5-methylenetetrahydrofolate. This reduction reaction creates methyl donor that plays a key role for the conversion of homocysteine to methionine [1]. Methionine synthase catalyze this conversion along with Vitamin B12 as a cofactor and this catalyst always coexist with Vitamin B12 in all mammalian tissues. Methionine is incorporated in dietary protein, and act as a predecessor of S-adenosylmethionine. When methionine is converted to homocysteine, S-adenosylmethionine acts as a methyl donor. Similarly in transsulfuration process the homocysteine can also be converted to cysteine through vitamin B6 dependent pathway [2].
The chromosome 1 at 1p36.3 possesses the gene that codes for 5, 10-Methylenetetrahydrofolate Reductase (MTHFR). This gene consists of 2.2 kilo bases long complementary DNA sequence and 11 exons [2]. 70-77 kDa subunits of dimeric proteins are encoded by this cDNA. The complementary DNA of human has the most catalytic activity and binding sites as compared to catalytic activity of porcine and bacterial enzymes [3,4].
Goyette P, et al. [2], Goyette P, et al. [4] and Daubner S, et al. [5] investigated by research work that there are 15 mutations of MTHFR gene that are associated with enzymatic deficiency, out of which 14 are infrequent and cause stern enzymatic deficiency and 1 is common that cause slighter enzymatic shortage. A point mutation at C677T in the MTHFR gene replaces the alanine to valine in the enzyme Rosenberg N, et al. [6], Kang SS, et al. [7] and Rozen R [8] examined that thermo stability of MTHFR enzyme is reduced due to this mutation and the enzyme show decreased activity at 37 ℃ or higher temperature. As compared to normal subjects the activity of MTHFR enzyme in homozygous subjects is reduced to 50-60% at 37 ℃ and 65% at 46 ℃.
The plasma Homocysteine (Hcy) level rise in the homozygous mutated subjects due to incapability of the MTHFR enzyme to convert 5, 10-methylenetetrahydrofolate into 5-methyltetrahydrofolate. The Hcy level is higher in homozygous mutated subjects and slightly elevated in heterozygous mutated subjects as compared to the normal subjects [8].
Methionine is only obtained by using the 5-MTHF as donor group. The use of homocysteine in the biochemical cycle is maintained by 5-MTHF, because rise in plasma Hcy level is associated with vascular injury that can start or speed up atherogenic and thrombotic reactions. Loscalzo J [9] reported that hydrogen peroxide and superoxide free radicals are formed quickly by oxidation of free Hcy in plasma, this free Hcy in plasma is able to injure the biological cellular membrane by oxidation, or it can initiate the per-oxidation reactions of lipoprotein particles in plasma.
In obese individuals the levels of circular oxidative stress markers are high because of processes involving pro-oxidant reactions for example inflammatory adipokine synthesis similar to leptin, TNF- α (Tumor Necrosis-Factor-alpha) and IL-β (Interleukine-1β) by macrophages and adipocytes. Obese individuals have enhanced respiratory action and less utilization of anti-oxidant molecules and vitamins [10]. It looks that in obese individuals little utilization of vitamins B12 and B6 and folic acid is linked with the existence of a larger prevalence of C677T of MTHFR that can be a factors responsible for low availability of cofactors and substrate necessary for the production of 5-MTHF.
Mojtabai R [11] established the relationship between low levels of folate and elevated body mass index. It is also assumed that obesity is influenced by folate levels through epigenetic control of the genes which regulate the body fat storage [12]. The methylation of DNA cytosine and histone amino acid residues is done with methyl groups provided by folate that may change the epigenetic gene expression [13]. The methylation of dinucleotides of gene involved in food intake, fat storage and cellular physiology or body weight will be affected by any defect in the genes that are involved in methyl group metabolism. Therefore, in the present study we hypothesized that the genetic polymorphisms 677CT of MTHFR gene may compromise the potency of MTHFR enzyme resulting in decreased levels of folate and associated with obesity. The particular objectives of the present study are to determine association of MTHFR C677T polymorphism with obesity in humans.
The present study included human normal and obese female subjects visiting various hospitals in Rawalpindi and Islamabad.
Inclusion criteria
All the subjects were from the same socioeconomic status and eating habits. The overweight subjects with minor illness like temperature, flu, and cough without any concurrent diseases and having obesity were recruited in the study after a written consent. The controls were normal weight female subjects taken from the same population.
Study protocol
Data collection: Data of socio-demographic, physical health and lifestyle aspects was collected in the form of a predefined questionnaire (Annex-I).
Measurement of Body Mass Index (BMI): Harpender Standiometer was used to measure the standing height close to 0.1 cm. Digital weight scale was used to determine the weight with a precision of 0.1 Kg. Following formula was used to calculate the body mass index.
BMI = Weight in kilogram
(Height in meter)2
The subjects with BMI of 18-25 normal weight and above 30 were considered obese.
DNA extraction: Standard phenol-chloroform method was used to extract DNA from blood samples. Extracted DNA was then stored at -20 ℃.
Visualization of extracted genomic DNA: Electrophoresis was used to determine the successful extraction of DNA. For this purpose 3μl of extracted DNA was used with bromophenol and run on 1-2% agarose gel on standard conditions with 100 Volts for 30-60 min in 1X TBE buffer. Ethidium bromide was used to visualize the DNA products, after electrophoresis. UV Transilluminator (300 nm) was used to detect the DNA.
DNA quality determination: A spectrophotometer reading at OD260 was used to quantify the Genomic DNA. DNA concentration was calculated based on the following formula:
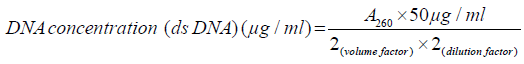
A ratio of light absorbance at 260nm to that at 280nm was used to determine the purity of DNA. A pure sample has a ratio equal to 1.8, a less ratio shows protein contamination while a greater ratio shows RNA contamination.
Primer design: Biotechnology Information (NCBI) sequences database (http://www.ncbi.nlm.nih.gov) was used to cμμμonstruct primers which were spanning the DNA flanking regions of MTHFR gene in order to get amplification of the entire sequence of the gene. Primer 3 programme was used to design primers (http://www.patch.com.ac.uk/cgi.bin/primer3.cgi). The specificity, dimmer and multiple priming sites were determined by using PCR simulation programme amplify 1.2. Primers were, chemically synthesized by a reputed company containing 20 base pairs with 50% GC contents.
SNP genotyping: The primers were optimized in specific PCR conditions. Genotyping of the SNP was performed accordingly. Restriction enzyme was used to digest the PCR amplified products to determine polymorphisms.
Gel electrophoresis: A 3% agarose gel electrophoresis was used to separate the digested product. The ethidium bromide was used as a stain that illuminates on ultraviolet transillumination and help in visualization of bands. The bands of fragments that were containing non-mutated and mutated alleles obtained on gel. The genotype and allelic frequency of the genetic polymorphism was obtained by directly counting bands on gel.
Statistical analysis: The mean ± S.E. of the quantitative variables was calculated. Chi-square analysis was used to determine the Hardy-Weinberg equilibrium of the alleles in the population. The association of the polymorphism with overweight condition and BMI was determined by logistic regression analysis adjusting the effects of confounding factors i.e. age, socioeconomic factors and lifestyle. A p-value<0.05 was considered statistically significant.
Association of lifestyle and medical factors with obesity
The mean age of obese (37.18 ± 1.232) were significantly (p<0.05) higher as compared to normal (31.50 ± 1.011) subjects. However, the multiple logistic regression analysis shows no significant (p<0.05) association between age and obesity (Table 1). Our data show that the female consuming extra meal (4th meal) are at a higher risk of obesity (OR: 11.104, 95% CI: 3.745-32.930, p<0.05). Obsessive eating also increases the odds (OR: 15.027, 95%CI: 3.435-65.739, p<0.05) of obesity. A routine daily walk and increasing sleep time have protective role (0.257, 95%CI: 0.126-0.525, p<0.05 and 0.652, 95%CI: 0.496-0.857) of obesity (Table 1).
| Factors | Control | Obese | OR (95% CI) | P value | AOR (95% CI) | p value |
|---|---|---|---|---|---|---|
| Age | - | - | 1.047 (1.019-1.075) | 0.001 | 1.025 (0.962-1.092) | 0.449 |
| Diabetes | 3 (3.0) | 25 (25.5) | 11.073 (3.219-38.091) | 0 | 20.944 (3.063-143.217) | 0.002 |
| Extra meal (4th meal) | 4 (4.0) | 31 (31.6) | 11.104 ( 3.745-32.930) | 0 | 25.061 (4.330-145.062) | 0 |
| Digestive disease | 1 (1.0) | 59 (60.2) | 149.769 (20.049-1.119E3) | 0 | 305.196 (30.864-3.018E3 | 0 |
| Goiter | 3 (3.0) | 9 (9.2) | 3.270 (0.858-12.461) | 0.083 | NC | - |
| Frequent fast food | 23 (23.0) | 30 (30.6) | 1.477 (0.784-2.783) | 0.228 | NC | - |
| Liver disease | 3 (3.0) | 39 (39.8) | 21.373 (6.322-72.254) | 0 | 18.483 (2.893-118.087) | 0.002 |
| Obsessive eating | 2 (2.0) | 23 (23.5) | 15.027 (3.435-65.739) | 0 | 18.341 (1.640-205.137 | 0.018 |
| Daily walk | 87 (87.0) | 62 (63.3) | 0.257 ( 0.126-0.525) | 0 | 0.064 (0.016-0.262) | 0 |
| Sleeping time | - | - | 0.652 (0.496-0.857) | 0.002 | 0.874 (0.472-1.617) | 0.667 |
| Family history of thyroid disorders | 3 (3.0) | 6 (6.1) | 2.109 (0.512-8.680) | 0.301 | NC | - |
We found an association between obesity and liver disease (OR: 21.373, 95%CI: 6.322-72.254, p<0.05). Digestive disease significantly (p<0.0) increases the odds ratio (OR: 149.769, 95%CI: 20.049-1.119) of obesity. There is also a significant (p<0.05) association of diabetes (OR: 11.073, 95%CI: 3.219-38.091) with obesity (Table 1).
Though, previous studies reported an association between age and obesity we did not find a significant association may be due to our age matched subjects. However, our results of association of obesity with an extra meal (4th meal) consumption and obsessive eating (a meal after every hour) and lack of physical activity are consistent with previous reports [14]. It is well established previously that the obsessive and frequent eating of carbohydrates and fat rich foods may increase the fats deposition and central obesity especially in elderly as anabolic lipolytic hormones such as Growth Hormone (GH) and sex steroids decline gradually with age 9 (Figure 1).
Figure 1. Representative gel picture for PCR amplification for MTHFR C677T. Product size 198bp. Ladder=100bp.
We have observed an inverse association between obesity and sleep time that is also reported previously. The values of BMI, body fat, waist and hip circumferences and fat mass index were higher for subjects with short sleeping time, especially for females [15-19]. The association between reduced sleep duration and obesity can be explained in different ways such as shorter sleep time leads to an excess of energy intake and lower energy expenditure as the awaking person tends to consume more and frequent food. On the other hand, insufficient sleep is associated with low anabolic lipolytic hormones (GH & sex steroids) and an anorexic (eating & fat deposition inhibiting) hormone, i.e. leptin. Appetite and hunger might be affected by this association, which leads to overeating and obesity. Hence in this way, the subjects with short sleep duration become obese because they have much time to eat and decreased energy expenditure due to fatigue and changed thermoregulation [20].
As in the present study, the association between obesity and digestive disease was also established in the previous study. The poor digestion, reflux and digestive ulcers may lead to central obesity. The liver diseases result in disorders of fat metabolism and lead to deposition of fats. The previous studies also reported the association between obesity and thyroid or goiter [21,22]. The hypothyroidism based goiter is associated with an increase in body weight and obesity [23]. It is also observed that obesity and insulin has a relation, but the status of cause and effect is dubious [24].
Association of Mthfr C677Twith obesity
Genotype distribution of the MTHFR 677 C>T polymorphism of the 198 subjects was analyzed: 117 (59. 09%) subjects were homozygous for the C allele (CC), 77 (38.89%) subjects were heterozygous (CT) and 4 (2.02%) subjects were homozygous for the T allele (TT). The minor allele (T) frequency of the MTHFR 677C>T polymorphism was 0.205, and the genotype distributions did not deviate from the Hardy-Weinberg equilibrium (p>0.05) (Table 2).
| Genotype | Obese n (%) | Control n (%) | OR (95% CI) | p value | AOR (95 % CI) | p value |
|---|---|---|---|---|---|---|
| CC | 57 (58.2) | 60 (60.0) | 1 | 1 | ||
| CT | 38 (38.8) | 39 (39.9) | 1.026 (0.577-1.823) | 0.931 | 0.739 (.217-2.518) | 0.628 |
| TT | 3 (3.1) | 1 (1.0) | 3.158 (0.319-31.247) | 0.325 | 20.002 (0.871-459.331) | 0.061 |
| CT+TT | 41 (41.8) | 40 (40.0) | 1.079(0.612-1.902) | 0.793 | 1.776 (0.287-2.954) | 0 |
| C | 152 (77.6) | 159 (79.5) | 1 | 1 | ||
| T | 44 (22.4) | 41 (20.5) | 1.123(0.695-1.814) | 0.637 | 1.182 (0.469-2.981) | 0.723 |
The alleles and genotype distribution were compared between obese (98) and control (100) subjects. Out of 98 obese subjects 57 (58.2%) were homozygous for C allele (CC), 38 (38.8%) were heterozygous (CT) and 3 (3.1%) were homozygous for T allele (TT). Out of 100 control subjects 60 (60.0%) were homozygous for C allele (CC), 39 (39.9%) were heterozygous (CT) and 1 (1.0%) was homozygous for T allele (TT). Furthermore, C allele was present in 152 (77.6%) obese and 159 (79.5%) control subjects. The frequency of T allele in obese and control was 44 (22.4%) and 41 (20.5%) respectively.
Allelic frequencies
According to the Table 2 the allelic frequencies were statistically similar (P>0.05) in the both groups of subjects suggesting lack of association between minor allele (T) and obesity.
Genotype frequencies
The genotypic frequencies were not significantly (p>0.05) different in obese and normal females. Therefore, no significant (p>0.05) association between MTHFR C677T and obesity was observed. Both the heterozygous (CT) (OR: 1.026, 95 CI: 0.577-1.823) and mutated homozygous (TT) (OR: 3.158, 95%CI: 0.319-31.247) increase the odds of obesity statistically non-significantly (p>0.05). However, the heterozygous and mutated homozygous (CT+TT) collectively showed a statistically significant (p<0.05) association (OR:1.776, CI: 0.287-2.954) with obesity, after adjusting odds ratios for age, diabetes, extra meal, digestive diseases, goiter, fast food frequent consumption, liver disease, obsessive eating, physical activity, sleeping time and family history of thyroid disorders.
The present study found no association between MTHFR C677T and obesity in human female obese population. However, it is observed that other factors of lifestyle habits and medical condition are responsible to cause obesity.
A number of studies have been conducted to investigate the association of MTHFR C677T polymorphism with obesity in various populations (Tables 3 and 4). Although, the results of most of these studies are similar that they didn’t find significant relationship between C667T and obesity [25-28], but there are two studies, i.e. Terruzzi I, et al. [29] and Yang B, et al. [30], a Caucasian and an Asian, respectively, that have shown a significant association between obesity and the polymorphism. Therefore, the differences in the results could be attributed to other factors such as variations in recruitment of subjects, sample size, ethnicity and geographic factors (Table 4). The results are also dependent on the general health, medical conditions (diabetes, liver diseases and digestive diseases, etc.) and lifestyle factors (frequency and type of food, sleeping and resting time and daily physical activity etc.), which were not addressed while selecting subjects in many of the previous studies [31-35].
| Cases | Controls | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Name | Country | Total | CC | CT | TT | CT+ TT | C | T | Total | CC | CT | TT | CT +TT | C | T | |
| Asian | Thawnashom K, et al. [27] | Thailand | 37/112* | 67 | 23 | - | - | 23/90* | 34 | 16 | - | - | ||||
| Bazzaz, et al. | Iran | 74 | 44 | 21 | 9 | 30 | 109 | 39 | 207 | 113 | 80 | 14 | 94 | 306 | 108 | |
| Fan SJ, et al. [20] | China | 517 | 115 | 244 | 158 | 402 | - | - | 741 | 160 | 375 | 206 | 581 | - | - | |
| Yin RX, et al. [28] | China | 378/373* | 354 | 341 | 56 | 397 | 1049 | 453 | 490/488* | 471 | 441 | 66 | 507 | 1383 | 573 | |
| Yang B, et al. [30] | China | 692 | 129 | 335 | 228 | 563 | 593 | 791 | 878 | 202 | 431 | 245 | 676 | 835 | 921 | |
| Caucasian | Terruzzi I, et al. [29] | Italy | 82 | 18 | 54 | 12 | 66 | 90 | 78 | 54 | 14 | 33 | 5 | 38 | 61 | 43 |
| Settin AA, et al. [26] | Saudi Arabia | 66/64* | 89 | 34 | 5 | 39 | 212 | 44 | 57/54* | 69 | 36 | 5 | 41 | 174 | 46 | |
| Gara S, et al. [24] | Tunisia | 31 | 15 | 14 | 2 | 16 | 44 | 18 | 22 | 9 | 12 | 1 | 13 | 30 | 14 | |
| Hernández-Guerrero C, et al. [25] | Mexico | 75 | 18 | 38 | 19 | 57 | 74 | 76 | 53 | 15 | 28 | 10 | 38 | 58 | 48 | |
| Lewis SJ, et al. [12] | UK | 882 | 360 | 410 | 112 | 522 | 1130 | 634 | 2534 | 1165 | 1086 | 283 | 1369 | 1130 | 634 | |
| Lewis SJ, et al. [12] | UK | 356 | 163 | 155 | 38 | 193 | 481 | 231 | 6135 | 2707 | 2713 | 715 | 3428 | 8127 | 4143 | |
| Lewis SJ, et al. [12] | Uk | 233 | 115 | 93 | 25 | 118 | 323 | 143 | 4897 | 2155 | 2190 | 552 | 2742 | 6500 | 3294 | |
| Lewis SJ, et al. [12] | UK | 1269 | 588 | 574 | 107 | 681 | 1750 | 788 | 7904 | 3812 | 3356 | 736 | 4092 | 10980 | 4828 | |
| Study [References] | Association | Sample Size (n) Obese/Control | Age | BMI | Folate Level | Hcy Level | Serum Vitamin B12 | Body Fat | Waist Circumference | Skin fold thickness | Blood Pressure | Triglyceride |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thawnashom K, et al. [27] | No | 149 Obese/113 Control | Yes | Yes | Yes | Yes | Yes | No | No | No | No | No |
| Bazzaz, et al. | No | 74 Obese /207 Control | Yes | Yes | No | Yes | No | No | No | No | No | No |
| Hernández-Guerrero C, et al. [25] | No | 75 Obese/ 53 Control | Yes | Yes | Yes | No | Yes | No | No | No | No | No |
| Lewis SJ, et al. [12] | No | 1858 Obese/ 18936 control | Yes | Yes | No | No | No | No | Yes | No | No | No |
| Fan SJ, et al. [20] | No | 517 obese/ 741 Controls | Yes | Yes | No | No | No | No | Yes | No | Yes | Yes |
| Terruzzi I, et al. [29] | Yes | 82 Obese/ 54 Control | Yes | Yes | Yes | Yes | Yes | No | No | No | No | No |
| Settin AA, et al. [26] | No | 130 Obese/ 111Control | Yes | Yes | No | No | No | No | No | No | No | No |
| Yin RX, et al. [28] | No | 751 Obese / 978 Control | Yes | Yes | No | No | No | No | Yes | No | Yes | Yes |
| Gara S, et al. [24] | No | 31 Obese / 22 Control | Yes | Yes | Yes | Yes | Yes | No | No | No | No | No |
| Yang B, et al. [30] | Yes | 692 Obese/ 878 Control | Yes | Yes | No | No | No | No | Yes | No | Yes | Yes |
Therefore, the present study was conducted to address the role of non-genetic factors along with MTHFR C677T polymorphism in obesity. The results reported by the present study, that there is no association between the polymorphism and obesity in spite of adjusting non-genetic factors are significant (Figure 2). However, there are few limitations in the present study that we could not measure folate and homocysteine levels of the subjects. A mutation in the MTHFR such as C677T may reduce conversion of homocysteine into methionine may lead to hyper-homocysteinemia that is found to be responsible for various health conditions such as cardiovascular diseases, infertility and obesity. Similarly, lower levels of folate were also found to be responsible for the obesity [35-43]. Therefore, a further study is needed to measure the folate and homocysteine levels in the obese patients along with MTHFR C677T.
We concluded that there is no association of MTHFR C677T with obesity in females. But, the extra meal (4th meal), obsessive eating, diabetes, digestive and liver diseases are among the major causes of obesity. However, a daily walk and increase in sleeping time have protective role. There are few limitations in the present study that we did not measure folate and homocysteine levels of the subjects. Therefore, a further study is needed to measure the folate and homocysteine levels in the obese patients along with MTHFR C677T.
Methylenetetrahydrofolate (MTHFR) is an enzyme that stimulates and modulates folate metabolism in the body. The main and specific function of MTHFR is to convert 5, 10- Methylenetetrahydrofolate into 5-Methylenetetrahydrofolate (5-MTHF), that is active folate. 5-MTHF is a key component in the synthesis of nucleotides for production of RNA and DNA by methylation reactions; production of S-Adenosyl Methionine (SAM); methylation of DNA, proteins, Neuro Transmitters (NTs), & phospholipids; and also the conversion of homocysteine into methionine by remethylation reaction. Many genetic variations in the MTHFR enzyme are recognized and reported which alter the folate metabolism hence methylation and overall health. Numerous studies have shown that lowering of folate level lead to the increased body mass index. Therefore, in the present study it was hypothesized that the genetic polymorphisms of MTHFR gene might be related to the obesity resulting in decreased levels of active folate. Hence, the methylenetetrahydrofolate SNPs may be associated with obesity. The objective of this study was to determine association of a specific MTHFR C677T polymorphism with obesity in human female obese subject. Samples of obese females were collected from various hospitals in Rawalpindi and Islamabad. Samples of controlled subjects (females having BMI<30.0) were also taken from the same population. Blood samples (~5 ml) along with the data of sociodemographic, physical health and lifestyle aspects were also collected. DNA was extracted from blood samples by using standard phenol-chloroform method and stored at -20℃. The specific primers were designed and optimized for the gene. The PCR amplified products were digested by a specific restriction enzyme to determine polymorphisms. Digested product was electrophoresed on agarose gel with ethidium bromide staining which was then visualized through ultraviolet transillumination. The fragments of wild type and mutated DNA were obtained on gel. The allele frequency of the C to T polymorphism was determined by counting allele through electrophoresis gel analysis. Chi-square analysis was used to determine the Hardy-Weinberg equilibrium of the alleles in the population. The association of the polymorphism with BMI was determined by logistic regression analysis adjusting the effects of confounding factors i.e. age, socioeconomic factors and lifestyle. A p value of <0.05 was considered statistically significant. We found no association of MTHFR C677T with obesity in females. But, the extra meal (4th meal) (OR: 11.104, 95% CI: 3.745-32.930, p<0.05), obsessive eating (OR: 15.027, 95%CI: 3.435-65.739, p<0.05), diabetes (OR: 11.073, 95%CI: 3.219-38.091), digestive (OR: 149.769, 95% CI: 20.049-1.119) and liver diseases (OR: 21.373, 95% CI: 6.322-72.254, p<0.05) are among the major causes of obesity. However, a daily walk and increase in sleeping time have protective role. There are few limitations in the present study that we did not measure folate and homocysteine levels of the subjects. Therefore, a further study is needed to measure the folate and homocysteine levels in the obese patients along with MTHFR C677T.
There are no acknowledgements.
Any financial interest or any conflict of interest exists.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Journal of Bioengineering & Biomedical Science received 307 citations as per Google Scholar report