Research Article - (2022) Volume 7, Issue 12
Received: 22-Jul-2020, Manuscript No. JIDM-20-15970;
Editor assigned: 27-Jul-2020, Pre QC No. P-15970;
Reviewed: 10-Aug-2020, QC No. Q-15970;
Revised: 30-Nov-2022, Manuscript No. R-15970;
Published:
28-Dec-2022
, DOI: 10.37421/2952-8097.2022.7.270
, QI Number: JIDM-20-15970
Citation: Tegegne, Awoke Seyoum and Awoke Fetahi. "Determinants for Hepatitis B Virus Seromarkers among Chronic Hepatitis B Patients under Medication Adherence: A Prospective Longitudinal Study." J Infect Dis Med 7 (2022): 270.
Copyright: © 2022 Tegegne AS, et al. This is an open-access article distributed under the terms of the creative commons attribution license which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Background: The natural background and treatment outcomes of hepatitis B virus infection are mainly affected by genotypes and viral load. In the world, millions of people are suffering from chronic viral hepatitis infections. The objective of current study was to identify factors affecting the hepatitis B virus seromarkers for people under treatment.
Methods: Prospective longitudinal study design was conducted for 409 chronic HBV patients. Linear mixed effect model was used in current investigation for data analysis. Estimation was done using restricted maximum likelihood technique.
Results: From the linear mixed effect model, main effects like visiting time (p-value<0.01), age (p-value<0.01), vaccination history (pvalue= 0.014), marital status (p-value=0.003), Alanine aminotransferase (p-value=0.006), Genotype (A, B, C) and Albumin (p-value=0.033) significantly affected the variable of interest. Similarly, interaction effects of time with marital status played statistically significance role for the progression rate of hepatitis B virus.
Conclusion: Aged patients, patients with elevated ALT, HIV infected patients, patients with genotype A, patients living with partners and patients who did not take vaccination in their childhood were groups identified in current investigation as at maximum risk and needs intervention. Evidences have been increased from time to time for certain population with chronic HBV infection being at great risk for their life. Hepatitis B virus infected patients at the study area should have information about factors potentially affecting the infection rate of the disease. Ministry of health or health staff should aware the community to take vaccination that helps to protect individuals from hepatitis B virus.
Hepatitis B patients • Infectious disease • Liver disease • Virus • Linear mixed model • Reml
ALT: Alanine Aminotransferase; HBV: Hepatitis B Virus; HIV: Human Immuno Virus; HCV: Hepatitis C Virus; REMLs: Maximum Likelihoods; AIC: Akakai Information Criteria; LRTs: Likelihood Ratio Tests; LMM: Linear Mixed Model; HBEAG: Hepatitis B E-Antigen; HBSAG: Hepatitis B Surface Antigen; HBCAG: Hepatitis B Core Antigen; NIH: National Institutes of Health.
Hepatitis B is the most widespread liver infection in the world. It is caused by the Hepatitis B Virus (HBV), which can attack and injure the liver. For many people in the world, the hepatitis B virus infection leads to a serious health problem with probably incurable health penalty. The very infectious virus particles in the blood of patients cause a severe health hazard with healthy asymptomatic carriers of the infection.
The National Institutes of Health (NIH) categorized chronic HBV infection in to three phases namely; the immune tolerant phase, the immune active phase and the inactive hepatitis B phase. In the first phase, HBV-infected persons are positive in HBeAg, have regular ALT levels and high levels of HBV DNA that are up to 20,000 IU/ML. In the second phase, infected people are described by very high ALT and HBV DNA levels with a minimum of 2000 IU/ML. Patients in the third phase (inactive hepatitis B phase) are described by the minimum level or absence of HBeAg and the occurrence of anti-HBe, ordinary ALT levels.
Experiences and symptoms of HBV vary greatly between infected people. Hence, about one‐third of them have sub‐clinical infection without any symptoms; one‐third experiences a mild “flu‐like” illness with symptoms like malaise, vomiting, nausea and soft fever and the remaining one‐third of them exhibit yellowing of the skin and eyes (jaundice), bilirubinuria, acute fatigue, anorexia, right‐sided upper abdominal pain and an inflamed tender liver.
About 90% of adults can be cured completely from the disease, although this may require at least six months adherent for the prescribed medication by the health staff and being free from drinking alcohol. For patients infected with hepatitis B virus, symptoms are visible in the blood and body fluids like semen and vaginal secretions of individuals (concentration about 1000 IU/ML). A diagnosis of patients with hepatitis B virus is therefore, based on the detection of the various viral antigens and antibodies in the blood or fluid. Some of the other diagnoses are conducted based on Hepatitis B surface Antigen (HBsAg), Hepatitis B core Antigen (HBcAg), Hepatitis B e- Antigen (HBeAg) and antibodies.
The virus can be transmitted from the patient to another individual through contact with infected body fluids and bloods or vaginal secretions of an infected person. The mode of transmission looks like HIV. However, HBV are 50 to 100 times more infection as compared to HIV. It can be transmitted at 3 stages in life; around the time of birth, during childhood, and in adult life. The virus must be introduced via mucous membranes or through broken skin for the infection to be occurred.
According to the WHO Global report, up to 2 billion people have been infected with HBV throughout the world; about 350 million people live with chronic HBV infection, and about 600,000 people die because of HBV-related liver disease every year. Hepatitis B shares a heavy burden on the health care system with high costs for treatment, failures for liver treatment and chronic liver disease. In many countries, hepatitis B is the leading cause of liver transplants. Treatments for infected people at the last stage are extremely expensive and unaffordable amount of money is requested for an individual to be cured. Hepatitis B is one of the major diseases of mankind and is a severe global public health problems. The risk of infection becomes chronic for 90% of infants, 30% of children under five and 2.6% for adults.
In Africa, infections related to chronic HBV play a major role in the etiology of most liver diseases. About 58 million people are living with the virus and 12.5 million died prematurely in African countries.
In Ethiopia, a national epidemiological study about hepatitis B virus prevalence was conducted on 5, 270 young males from all regions of the country. Overall prevalence rate was 10.8% for HBsAg and 73.3% was at least one marker positive. A remarkable geographical and ethnic variability of marker prevalence was observed and this reflects the wide variation of socio-cultural, environment, tribal practices and traditional surgery. Genderual practice and medical exposure also play a major role as determinants of hepatitis B marker prevalence and chronic viral hepatitis also results in loss of productivity.
Because of the non-symptomatic behavior of the infection of chronic HBV, people are often unaware of the fact that they are infected with the virus, unless they are regularly checked or aware about predictors for the prevalence of the disease.
Different studies conducted so far, lead us to consider that different genotypes linked with different rates of development from acute to chronic HBV infection. However, different hosts and environmental factors make certain difficulties to identify findings from one geographical region to another. Hence, there are inconsistencies of findings obtained from different investigations conducted previously. Some of the researches conducted previously were crosssectional (single observation taken from each individual) and there was no chance of observing repeated observations on the same subject. One of the previous research recommended that it is important to conduct a well design, prospective longitudinal study to identify valid predictors of hepatitis B virus genotype for chronic hepatitis B patients in which it helps to guide treatment strategy of the disease. Therefore, the current investigation was conducted with objective of identifying the risk factors for hepatitis B virus genotype for hepatitis B patients under medication [1].
Study area and population under investigation
The research was conducted at Felege Hiwot teaching and specialized hospital, Amhara region, North-west Ethiopia. The hospital serves as teaching, specialized and referral for many district hospitals in the region. Many people infected with hepatitis of any type at many districts are referred to this hospital and get treatment including transplantation of liver in the region. Hence, a total of 2035 hepatitis B virus patients were targeted as study population [2].
Study designs
A prospective longitudinal study design was conducted on people infected with hepatitis B virus who were followed up their treatment for 36 months in the hospital as outpatients.
Source of data
The current investigation includes secondary data about social, demographic and clinical characteristics of 409 people infected with hepatitis B virus.
Sample size and sampling procedures
Among all people infected with HBV, a purposive sample of 409 patients who had full viral load records at every follow-up time were included in the investigation. The available data at the hospital was first observed and discussed with health care service providers. All relevant information from the cards of patients was collected by health care service providers after theoretical and practical orientations about variables under investigation.
Data collection tools
Data was extracted using data extracted format developed by the investigators in consultation to health care providers. Charts of patients with full viral load records were retrieved using patients’ registration card number. The electronic data base system was used in extraction of data from treatment center [3].
Quality of data
The quality of data was controlled by data quality controllers from the specific section of the hospital and regional laboratory center; where patients’ viral load diagnosis was conducted. The data extraction tools and variables under investigation were pretested for consistency of understanding and completeness of the required information on 50 hepatitis B virus patients before final data collection. Based on the information from the pilate test, necessary amendments were made on the final data collection format. The retrieval process in data collection was closely monitored by the investigators in the whole data collection period. Both the predictor and variable of interest were checked regularly for their completeness of information. Any inconvenience related to data collection was immediately communicated to data collectors and corrective measures were taken.
Patients and their treatment procedures in the hospital
All patients registered at the hospital were followed up for six months to assess treatment outcomes. Based on the six months treatment outcomes, patients were classified as: Cured (finished treatment with negative bacteriology) or uncured (finished treatment without negative bacteriology). Patients who fished treatment with negative bacteriology provided with free HBV medications certificate after end of their treatment (end of six months). If patients, at the end of the 6th month follow ups are not cured, they are referred as chronically infected patients. Then after, patients were directed to visit the hospital every quarter (three months) for further medication follow up and checkup for the HBN DNA markers.
Inclusion criteria
Hepatitis B patients identified as chronically infected with HBV after six months follow ups, patients with full viral load records at each visiting time and whose follow ups from January 2013 to December 2016 were included in the investigation [4].
Variables included in current investigation
The response variable: The response (dependent) variable was expected HBV DNA marker expressed in IU/ML. It measures the number of HBV DNA and the higher the number indicates how rapidly the virus is reproducing it self in the liver and how many HBV DNA units are found in a milliliter or about one drop of blood. The higher the number also indicates the severity of the disease.
Predictor variables: predictor variables under current study were Gender (Male, Female), Age in years, Con infection with HIV (Yes, No), Co infection with any other disease (Yes, No), Marital status (Living with partner, Living without partner), History of vaccination (No, Yes), Baseline ALT level (Normal, Elevated), HBV with genotype A (No, Yes), HBV with genotype B (No, Yes), HBV with genotype C (No, Yes), HBV with genotype others (No, Yes), albumin level and number of visiting times [5].
About the model: A Linear Mixed effect Model (LMM) is a parametric model for longitudinal or repeated measures that quantifies the relationships between a continuous dependent variable and various predictor variables. The model includes both fixed effect parameters associated with one or more continuous or categorical covariates and random effects associated with one or more random factors. Fixed effect parameters describe the relationships of the covariates to the dependent variable for entire population; whereas, random effects are specific to subjects within a population. Consequently, random effects are directly used in modeling the random variation in the dependent variable at different levels of the data.
The linear mixed effects model assumes that the observations follow a linear regression where some of the regression parameters are fixed or the same for all subjects, while other parameters are random or specific to each subject. Meanwhile, population parameters, individual effects and the within subject variations make up the first two stages of the model. The general form of the linear mixed effects model after combining the two stages is approximately normal. The two stage of the model are indicated as follows [6].
First stage of the model belongs to individual response Yij for ith subject measured at time tij, i=1,---, n: j=1,---, ni response vector Yi for ith subject:
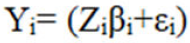 (1)
(1)
Where, Yi= (Yi1, Yi2, ---, Yin1)T
Zi is a ni × q matrix of known covariates, βi is q dimensional vector of subject specific regression coefficients and εi~(0, ∈i), often ϵi=α2 Ini, describes the observed variability with in subjects.
Second stage describes the between subject variability that explains in the subject specific regression coefficients using known covariates.
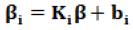 (2)
(2)
Ki is a q × p matrix of known covariates, β is a P dimensional vector of unknown regression parameter bi~N (0, D). Combining the two stages (1) and (2) provides:
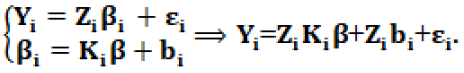 (3)
(3)
Let Xi=Zi Ki in (3), then the general form of the model is expressed as:
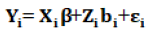 (4)
(4)
Where Yi represents a vector of continuous responses for the ith subject, Xi is an ni × p design matrix in the fixed effect, which represents the known values of the p covariates.
Methods of model selection was carried out using AIC, BIC and other information criterion, assuming the model with smallest information criteria as the best one. After final model selection, the model was refitted using REML estimation methods and restricted maximum likelihoods (Wald Test) were used for parameter estimation [7].
Likelihood Ratio Tests (LRTs) were used to compares two models, the full model with all of the interactions and the reduced model with just a subset of terms. The variance covariance structures were compared and selected using information criterion with the lower value to be considered as an appropriate model [8].
The medical cards of 409 patients had been reviewed, of which 57.9% of patients were moderate (>1000 IU/mm3) and 42.1% of patients were chronic (>2000 IU/mm3) level of HBV replication in their liver. All patients visit the hospital 10 times and had HBV values for each visit. Hence there was no missing value. The average (median) age of all patients was 38 with IQR (29, 41) years. Among the patients, 47.4% of them had drug history, 55.3% had elevated ALT. Among HBV patients, 13.2% [9].
Were co-infected HIV and 42.1% of them were living with their partners. The base line characteristics of study participants for current investigation are given in Table 1.
| Variables | Categories | N (%) |
|---|---|---|
| Age | - | 38 (29, 41) year |
| Visiting times | - | 10 visits |
| HBV level | Moderate (HBV>1000 IU/mm3) | 237 (57.9) |
| Chronic (HBV>2000IU/mm3) | 172 (42.1) | |
| Gender | Male | 215 (52.6) |
| Female | 194 (47.4) | |
| Co-infected with HIV | Yes | 54 (13.2) |
| No | 355 (86.8) | |
| Co-infected with any other disease | Yes | 176 (43.0) |
| No | 233 (57.0) | |
| History of drug use, HDU | Yes | 194 (47.4) |
| No | 215 (52.6) | |
| ALT | Elevated | 226 (55.3) |
| Normal | 183 (44.7) | |
| Marital status | Living with partner | 172 (42.1) |
| Living without partner | 237 (57.9) | |
| Genotype | A | 60 (14.7) |
| B | 108 (26.4) | |
| C | 95 (23.2) | |
| Others | 146 (35.7) | |
| Adherence to medication | Adherent | 194 (47.4) |
| Non-adherent | 215 (52.6) | |
| Drinking alcohol | Yes | 95 (23.2) |
| No | 364 (76.8) |
The data for its normality was assessed using the Q-Q plot. The Q-Q plot indicates that the ordered data of the observed value versus the expected normal probability satisfied the assumption of normality. The normality assumption was also checked for random effects and the random term was normally distributed [10].
Certain covariance structures were compared to select the one appropriate to the given data. Some of the covariance structures used for model selection was; Unstructured (UN), Compound Symmetric (CS), first order Autoregressive (AR (1)) and Toeplitz (Toep). Table 2 displays the corresponding fit statistics of the structures obtained from the newton raphson algorithm.
| Information criterion | Un | CS | Teoplz | AR(1) |
|---|---|---|---|---|
| AIC (smaller is better) | 53521.6 | 50204.6 | 54079.9 | 49835.5 |
| AICC (smaller is better) | 53521.6 | 50204.6 | 54080.5 | 49835.5 |
| BIC (smaller is better) | 53517.6 | 50194.6 | 54029.9 | 49827.5 |
| Average CCC | -0.2341 | -0.671 | 0.4904 | 0.9709 |
Restricted maximum likelihood was used for parameter estimation of type 3 and multivariate data analysis. The parameter estimates for type 3 tests is indicated at Table 3.
| Effect | DF | F Value | pr>f |
|---|---|---|---|
| Visiting times | 1 | 253.28 | <0.0001 |
| Gender | 1 | 0.12 | 0.1732 |
| Age in years | 1 | 28.38 | <0.0001 |
| HDU | 1 | 0.11 | 0.1741 |
| ALT | 1 | 7.67 | 0.0057 |
| Co-infected with HIV | 1 | 3.24 | 0.0231 |
| Co-infected with any other disease | 1 | 4.24 | 0.0321 |
| Mar. status | 1 | 12.81 | <0.0001 |
| Genotype | 3 | 0.88 | 0.0449 |
| Albumin | 1 | 4.56 | 0.0329 |
| Time marital status | 1 | 13.51 | <0.0001 |
| Time genotype | 3 | 11.94 | <.0001 |
| Time albumin | 1 | 9.27 | 0.0024 |
Significant predictor covariates at 0.25 level of significance from Table 3 were selected for further analysis in fixed and random components of the linear mixed effect model used for data analysis. Hence, all covariates at 0.25 level of alpha were significant. The multivariate data analysis is indicated in Table 4.
| Effect | Estimate | Standard error | t-value | Pr>t |
|---|---|---|---|---|
| Intercept | 54.32 | 3.78 | 11.4 | <0.001 |
| Time | -2.9 | 2 | -7.26 | <0.001 |
| Gender (Reference=Male) | ||||
| Female | 5.54 | 4.62 | 0.34 | 0.1732 |
| age | 3.59 | 7.87 | 5.33 | <.0001 |
| Vaccination for HBV at child age (Reference=No) | ||||
| Yes | -5.74 | 1.02 | -0.33 | 0.0141 |
| ALT (Reference=Normal) | ||||
| EL | 4.24 | 1.45 | 2.77 | 0.0057 |
| Co-infected with HIV(Ref.=No) | ||||
| Yes | 3.47 | 4.36 | 1.78 | 0.0434 |
| Marital status (Reference=living without partners) | ||||
| Living with partners | 2.72 | 2.56 | 2.95 | 0.0032 |
| Genotype (Reference=other) | ||||
| A | 6.03 | 1.25 | 3.03 | 0.0154 |
| B | -2.91 | 1.67 | -1.33 | 0. 0183 |
| C | -3.77 | 2.24 | -0.46 | 0.0143 |
| Albumin | -3.4 | 3.66 | -2.14 | 0.0329 |
| Adherence to medication (Ref.=Non-adherent) | ||||
| Adherent | -1.24 | 1.52 | -2.33 | 0.0121 |
| Drinking alcohol status (Ref.=No) | ||||
| Yes | 0.47 | 1.36 | 0.78 | 0.0414 |
| Time marital status (Reference=Living without partner) | ||||
| Living with partner | 3.94 | 6.22 | 2.86 | 0.0042 |
As it is indicated in Table 4, as visiting time increased by one unit, the expected number of HBV DNA marker in the droplet of blood was decreased by 2.9 (t-value=-7.26, p-value<0.001) given the other variables constant. Hence, on average the replication of hepatitis B virus inside the liver of patients was decreased with increase of follow up treatment [11].
However, as age of patients increased by one year, the expected hepatitis B virus DNA marker was increased by 3.59 IU/ML of blood (t-value=5.33, p-value<0.001), given the other things constant.
The expected number of HBV DNA marker for patients co-infected HIV was increased by 3.47 IU/ML of blood (t-value=1.78, pvalue= 0.0434) as compared to HBV patients free from HIV, keeping the other variables constant [12].
The expected number of HBV DNA marker for patients with elevated ALT (Amino Liver Transfers) level was increased by 4.24 IU/ML of blood (t-value=2.77, p-value 0.0057) as compared to that of a patient with a normal ALT level, given other things constant.
The expected number of HBV DNA marker for patients with genotype A was increased by 6.03 IU/ML of blood (t-value=3.03, pvalue= 0.0154) as compared to that of the patients with other genotypes, however, the expected number of HBV DNA unite for patients with genotype B was decreased by 2.91 IU/ML (tvalue=- 1.33, p-value=0.0183) as compared to patients with other genotypes. Similarly, the expected number of HBV DNA units for patients with genotype C was decreased by 3.77 IU/ML (tvalue=- 0.46, p-value=0.0143) as compared to other genotypes. The expected number of HBV DNA marker for patients living with partners was increased by 2.72 IU/ML (t-value=2.95, p-value=0.0032) as compared to patients living without partners. As the amount of Albumin in hepatitis B patients increased by one unit, the expected number of hepatitis B virus was decreased by 3.4 IU/ML (tvalue=- 2.14, p-value=0.0329), keeping the other things constant. The expected number of HBV DNA marker for adherent to medication patients was decreased by 1.24 IU/ML (t-value=-2.33, pvalue= 0.0121) as compared to non-adherent to medication patients [13].
The expected number of HBV units for patients who took vaccination at their childhoods was decreased by 5.74 (t-value=-0.33, p-value=0.0141) as compared to those patients who did not take vaccination. The interaction effect of time with marital status had significant effect on current investigation.
Hence, as visiting time of a patient increased by one unit, the expected number of HBV DNA unit for patients living with partners was increased by 3.94 IU/ML (t-value=p-value=0.0042) as compared to those patients living without partners [14].
For those patients, who strictly followed their medication ordered by health professionals declined the expected number of HBV. This indicates that the disease can be cured using appropriate adherence of the prescribed medication by the health staff, keeping other external things constant. Hepatitis B virus infected individuals, who adhered the prescribed medication, achieved and maintained undetectable HBV unit. This result is consistent with previous investigation [15].
Patients who had taken vaccination during child hood had low expected number of HBV as compared to those who did not take vaccination. Hence, vaccination at child hood had a power of reducing viruses’ replication and medication makes undetectable number of HBV. This result is consistent with one of the previously conducted research [16].
Treatment of hepatitis B virus for patients who are also con infected by HIV complicates the supervision of the progression rate of HBV infected individuals. On the one hand, individuals who are coinfected by hepatitis B virus should have regular liver-related measures such as hepatocellular carcinoma as compared to HBV mono infected individuals. On the other hand, an enlarged the risk of hepatotoxicity in individuals infected with HIV, the use of antiretroviral agents to the care for HIV related immunodeficiency becomes challenged for chronic hepatitis B. Hence, the resistance of medication by HBV leads for high progression of viral load [17].
In HIV infected individuals with high number of hepatitis B virus and low CD4 cell counts are associated with HBV viremia. An earlier introduction of antiretroviral therapy and anti HBV agents for HIV/ HBV-co-infected individuals may help for the reduction of the progression rate of the two viruses. The HBV viremia is connected with the risk of liver-related complications and HBV drug resistance [18].
Marital status has significant association with the progression of HBV in such away that patients living with their partners has high expected number of HBV because of their Genderual intercourse makes infection and replication of the virus itself. This result is consistent with one of the previous researches and contradicted with another previously conducted research. The reason indicted in the research opposed to a result in current investigation is that partners living together may encourage the patient to be adherent for the prescribed medication by the health staff. Hence, this result needs further investigation [19].
As visiting times of patients increased, the progression rate of hepatitis B virus decreased and becomes undetectable for patients living without partners as compared to those patients living with partners. The reason for this, might be HBV infected patients living with partners make Genderual intercourse and this makes infection and facilitates the replication of the virus [20].
The result under this investigation indicates that, age, genotype, history of co infection, whether or not patients took vaccination at childhood and follow up times played significant role for the progression rate (replication of HBV DNA unit). Therefore, more attention should be given for aged patients, for HBV infected patients co infected with other diseases like HIV, for individuals who did not take vaccination at childhood and ALT elevated patients. The health care workers should always measure the baseline Alanine Aminotransferase (ALT) level and the baseline hepatitis B virus deoxyribonucleic acid of patients and must know the baseline age of patients when they come to the hospital for the first time. This helps in control of the replication of the virus inside patients’ liver. The health staff at health institution should also register full personal information of patients at each visiting time/follow up times and should apply different screening techniques for patients infected with HBV. Finally, Policy makers should revise and strengthen vaccination programs for children at their childhood. One of the strengths of current investigation is that as far as our knowledge is concerned, the it is the largest virologic description of HBV DNA unit conducted so far in study area. The study consisted of longitudinal or repeated observations without any missing value on each individual which provides a chance of observing repeated response from each subject and this makes stronger the validity of results on each patient. This study was not without limitation; one such limitation is that the sample was taken in one treatment center (referral hospital). Taking samples from different centers and including all potential other predictors may have additional information for current investigation. Further investigation including all other potential cases for progression rate of hepatitis B virus which are not included under current investigation are recommended as potential gaps for future investigation.
Ethics approval and consent to participate: Since the data applied in current investigation was secondary, there was no need formal ethics approval. However, the ethical approval committee of Bahir Dar University approved to use the secondary data and provided an ethical clearance certificate for authors with Ref ≠ RCS/1412/2013. It is possible to attach the ethical clearance certificate upon request.
The manuscript did not publish anywhere and is not under consideration for publication. Finally, the authors agreed for the manuscript to be submitted to this journal for publication as original research.
The secondary data, which is available with the corresponding author, will not be made available publicly due to concerns about protecting participants’ identity and respecting their rights to privacy.
There is no conflict of financial non financial interest between authors or between authors and institutions.
Not applicable.
AF wrote the proposal, develop data collection format, supervise the data collection process, analyzed and interpreted the data. AS participated in proposal preparation, design and data analysis and critically read the manuscript and gave constructive comments for betterment of the manuscript. All authors are contributed on manuscript preparation
Amhara Region Health Research and Laboratory Center at Felege +Hiwot Teaching and Specialized Hospital and all health staffs are gratefully acknowledged for the data they supplied for current health research. A medical doctor medical college of Bahir Dar university is also highly acknowledged for his proper description of medical terminologies under this investigation.
Crossref, Google Scholar, Indexed at
Crossref, Google Scholar, Indexed at
Crossref, Google Scholar, Indexed at
Crossref, Google Scholar, Indexed at
Crossref, Google Scholar, Indexed at
Crossref, Google Scholar, Indexed at
Crossref, Google Scholar, Indexed at
Crossref, Google Scholar, Indexed at
Crossref, Google Scholar, Indexed at
Crossref, Google Scholar, Indexed at
Crossref, Google Scholar, Indexed at
Journal of Infectious Diseases and Medicine received 1059 citations as per Google Scholar report