Research - (2021) Volume 10, Issue 8
The present work highlights the influence of gamma-radiation on Cu2InSnS4 (CITS) thin films deposited by spray pyrolysis technique. Irradiation treatment was carried out by different doses (20-40-60-80 and 100 kGy) of γ-radiation using Co60 as source. The physical investigations of samples were demonstrated using energy dispersive X-ray spectrometry (EDX), X-ray diffraction (XRD), Maud software, scanning electron microscopy (SEM), Spectrophotometer and Drop Shape Analysis System. Firstly, XRD patterns reveal a decrement in peak intensities followed by the division of peaks related to (204) and (312) lattice plans after gamma-radiation. All films were crystalized into stannite structure and the crystallites were orientated towards (112) plan. Secondly, EDX spectroscopy reflects an appreciably decrease in Cu, In, Sn and S contents. SEM micrographs clearly show a total morphological modification from nanospherical to pyramidal shapes at 60 kGy, hierarchical rods and lamellar shapes at 100 kGy. Therefore, a special emphasis has been focused on surface wettability of irradiated films, which point out the hydrophilic surface after irradiation. As known, hydrophilic character has a notable beneficial role on photocatalytic activity that may be due to the active surface area and the adsorption of dye. Based on the relationship between hydrophilicity and photocatalysis, we have confirmed experimentally the better capacity of irradiated CITS thin film with 60 kGy to decompose Rhodamine B (RhB) dye under Xenon irradiation. Long-term runs confirm the stability of irradiated CITS with 60 kGy for photocatalytic process after an overall duration for 5h:30 (4 cycles of 120 min each). This result demonstrates that irradiated CITS with 60 kGy may considered as an efficient stable photocatalyst for the remediation of water polluted.
• Cu2InSnS4 thin film • Spray pyrolysis • Gamma-radiation • Hydrophilic surface • Recyclable photocatalyst
Continued advancements in quaternary chalcogenides require the development of their properties not only for photovoltaic, but also for photocatalytic, self−cleaning surfaces, antifogging and so on [1-3]. Various quaternary chalcogenides have been studied for this purpose, such as Cu2FeSnS4, Cu2ZnSnS4, Cu2CoSnS4, and Cu2NiSnS4, etc [4-7]. Recently, these derivatives chalcogenides are similar alternative absorbers with direct band gap in the range of (1-1.5) eV, absorption coefficient higher than 104 cm-1 and good electrical resistivity around 5.82 10-3 Ω.cm [8]. Thus, they would be bright candidates for utilization in optoelectronic applications. Recently, we have prepared new chalcogenide Cu2InSnS4 with optimal energy band gap about 1.4 eV, suitable electrical resistivity of 5.95 10-3 Ω.cm with hydrophobic surface.
Exposing materials to high gamma-radiation can enhance their semiconductor performances owing to its strong capacity to pass through the material with longer distance [9]. Gamma-radiation was used for various applications such as dosimeter, calibrations and sterilization of food products [10-12]. Numerous studies have been focused to investigate the influence of the interaction between γ-radiation and materials like InP, CoPc, CuInSe2 and Se1-xTex [13-16]. Structural properties of SnO, InSe and Cu2ZnSnS4 materials have been enhanced after γ-radiation [17-19]. Moreover, CoPc and InP semiconductors undergoes a degradation in their crystallinities [13,14]. It was mentioned that electrical resistivity was decreased for In2O3 [10], Al doped ZnO [9] and SnO [17] materials after exposing to high γ-rays. A few progresses have been made for the effect of gamma-radiation on physical properties of quaternary chalcogenide. Particularly, Cu2ZnSnS4 thin film was irradiated by10, 20, 30, 40, 50 and 100 kGy in our previous research [19].
The wetting behavior was inspected as a very important property from both practical and fundamental point of view. Surface wettability is deduced from water contact angle θ (WCA), which determined by the angle between solid/air and liquid/air interfaces. Based in literature, hydrophilic character obtained when the liquid wet the solid surface with θ<90°. When the liquid does not wet the solid surface with θ>90° it has hydrophobic character and super-hydrophobic character when θ>150° [20]. On the other hand, hydrophobicity and super-hydrophobicity possess extensive applications scale like self-cleaning, anti-corrosion and friction reduction [21,22].
Currently, photocatalysis process was applied for contaminant treatment to remove organic pollutants that considered dangerous for human health and harmful to environment [23]. Quaternary chalcogenides with narrow band gap are considered as suitable candidates due to their high ability to absorb light in Vis-NIR regions. In this paper, we have exanimated the influence of γ-rays on Cu2InSnS4 (CITS) thin films by 20-40-60-80 and 100 kGy. In particular, this work highlights the investigation of surface wettability of CITS thin films before and after γ-irradiation. Additionally, photocatalysis treatment was carried out by testing the degradation of Rhodamine B (RhB) dye after four recyclable process.
Synthesis of Cu2InSnS4 (CITS) thin layers and gammairradiation
Cu2InSnS4 thin films were grown using ‘Spray pyrolysis’ technique. Chemical constitutions with desired amount of copper (II) chloride dehydrate (CuCl2, 2H2O), indium chloride dehydrates (InCl3, 3H2O), tin (II) chloride dehydrates (SnCl2, 2H2O) and Thiourea (CH2N2S) were dissolved in 900 ml of methanol separately with 0.01/0.004/0.004 and 0.04 M respectively by continuous stirring for 10 min. All chemical products that used to synthesis CITS were bought from Fluka and Sigma Aldrich. After well mixing chemical products, Cu2InSnS4 solution was pulverized gradually as fine droplets with flow rate=25 ml/min using compressed air as carrier gas. This solution was sprayed onto glass substrates which heated under Ts=240°C. An industrial gamma irradiator composed by cobalt source (Co60) was used to irradiate CITS thin layers at various γ-doses 20-40-60-80 and 100 kGy in ambient air and room temperature.
Photocatalytic activity of irradiated Cu2InSnS4 (CITS) thin layers
Photocatalytic process of irradiated CITS was studied by the degradation of aqueous Rhodamine B (RhB) solutions under Xenon irradiation. Accordingly, RhB powders were dissolved in 1L of bi-distilled water to make the concentration of 3.10-5 mol/L and stirred for five hours. Therefore, the samples were added in 30 mL of RhB solutions in dark. In fact, when RhB molecules are adsorbed in film surface, they will react rapidly with electronholes pairs. When the adsorption-desorption equilibrium was reached, all RhB solutions which contain the films were placed under irradiation light for photocatalytic process. Then, the RhB degradation treatment was carried out under Xenon irradiation by a cylindrical light with a power of 55 W. The distance from the incident source light-solution was 12 cm. In the end, the photocatalystic degradation of RhB dye was carried out during 30, 60, 90 and 120-min. UV-Vis spectrophotometer type Perkin Elmer Lambda 950 was used to determine the absorbance values of dye solutions. After the first photocatalysis process (1st cycle), irradiated CITS photocatalyst were recovered, washed, dried in a furnace at 80°C and reused in a new RhB solutions under the same conditions. The recyclability of irradiated Cu2InSnS4 photocatalyst was also studied for other three cycles (2nd, 3rd and 4th cycles)
Wettability measurements
The surface wettability of irradiated Cu2InSnS4 thin films were performed from Water Contact angle θ (WCA) measurements using Drop Shape Analysis System type DSA100 (Kruss GmbH, Hamburg) as show in Figure 1. WCA was measured on the horizontal of material surface and carried out from scanning of profile images of water drops. The sample was placed under the point of a blunt−end needle. The needle was straightly connected to a micro−syringe pump that controlled by a computer. The measurements were studied using deionized water drop (~5 μl) on film surface. Water contact angle value was calculated from an average of the left and right drop angles. For more precision, the measurement of water drops was retreated on three different sites. Then the final WCA value was calculated from the average of acquired values. Mechanical vibration and Airflow were averted during the evaluation,
Characterizations
For clarity, element composition was determined using energy dispersive X-ray spectroscopy (EDX). Surface morphology was obtained by scanning electron microscopy (SEM) images using a Zeiss EVO MA10 microscope. XPERT-PRO diffractometer system over 10°-70° by using Cu-Kα radiation (λ=1.5418; 45 kV), was carried out to investigate structural behavior. Experimental XRD spectra were fitted by MAUD software (Materials Analysis Using Diffraction) which is based on Rietveld analysis and modeled by analytical functions. Optical properties were assessed by spectrophotometer type Perkin Elmer Lambda 950 in the wavelength range of (250-2500) nm
Chemical composition
EDX was used to determine the element compositions of Cu2InSnS4 thin films and whose results were described in Figure 2. It is clearly seen that all element compositions were decreased after exposure to γ-rays. Irradiated CITS by 60 kGy was Cu-rich content. This trend can reflect to form secondary phase in current with Cu2InSnS4 phase. 100 kGy of gamma-dose keeps the stoichiometric ratio of Cu:In:Sn:S near to 2:1:1:4. It is acknowledged that CITS structure was formed by sulfur, copper, indium and tin, where sulfur has the lowest binding energy and atomic radius compared to other elements. In addition, γ-energy (E=hν=P2/2 m) with 100 kGy may result ionization of sulfur and move it from surface into crystal lattice. Therefore, it may generate an excess of sulfur content [19].
Structural characterization
Figure 3 presents the XRD patterns of non-irradiated (0 kGy) and irradiated CITS thin films by 20-40-60-80 and 100 kGy. XRD diffractogramme of nonirradiated CITS thin layer showed that the peaks located at 2θ=28.71°, 47.59° and 56.54° were indexed to (112), (204) and (312) lattice plans. These main diffraction peaks are well matched to the standard (JCPDF card No. 74-1025) which correspond to stannite structure with I4 ̅2 m space group. As observed from Figure 3, the peak intensity of preferred orientation (112) lattice plan was decreased, suggesting a structural disorder of crystalline quality created after irradiation. It’s known that gamma-radiation produces structural defects such as: color centers (oxygen vacancies) or impurities, which have been deeply affect the crystallographic aspects like the appearance of secondary phases, a shift in diffraction angles and changes in peak intensities [19]. Similar results are shown by other authors [14-24]. Figure 3 and Table 1 reveals a slight shift of preferred orientation (112) lattice plan to lower angles. This finding is believed to the consequence of unit cell volume expansion (as demonstrated below), changes in stoichometric composition as observed in Figure 2 and microstructure parameters (as proved below). From XRD analysis, it is obvious that the peaks related to (204) and (312) lattice plans were divided on two peaks after gammaradiation. This trend can be explained as: Exposure to high γ-radiation may causes also moving atoms from their sites in order to occupy other sites, which lead to change the crystalline quality [24].
| X-ray diffraction result | MAUD software | |||||||
|---|---|---|---|---|---|---|---|---|
| g-doses (kGy) | D(nm) | a (A) | c (A) | E *10-4 | D(nm) | a (A) | c (A) | r.m.s*104 |
| 0 | 24 | 5.43 | 10.67 | 14.1 | 22 | 5.49 | 10.82 | 13 |
| 20 | 9 | 5.20 | 11.93 | 37.6 | 7 | 5.23 | 11.85 | 196 |
| 40 | 12 | 5.46 | 10.89 | 28.2 | 11 | 5.46 | 10.79 | 82 |
| 60 | 28 | 5.61 | 10.41 | 12.1 | 27 | 5.51 | 11.02 | 10 |
| 80 | 21 | 5.63 | 10.29 | 16.1 | 21 | 5.62 | 10.15 | 46 |
| 100 | 18 | 5.40 | 11.26 | 19.0 | 17 | 5.43 | 11.08 | 58 |
Crystallite size (D), dislocation density (δdis), the number of crystallites (Nc) and the strain (ε) are determined by the following formula [25] :
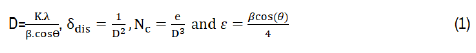
Where: λ is the wavelength of incident X-ray, k=0.9, β is the full width at half maximum, θ is the Bragg angle and e is the film thickness. After γ-radiation, the crystallite size of CITS thin layer was decreased from 46 nm to a minimum value of 9 nm for 20 kGy. This behavior may be due to the strong capacity of γ-rays to penetrate through the targeted film, which can displace atoms and breakdown the molecules that can reduce the crystallite size into smaller one. The analogous trend was previously reported in our researcher for iron doped indium oxide [24]. The observed increment of crystallite size at 60 kGy may be related to the gamma radiation nature that ionizes the matter and gives velocity through atoms. Therefore, it will generate multiple ions and electrons to atomic collisions [19]. Generally, δdis and Nc were increased after applying γ-rays reaching respectively 12.34 1012 cm-2 and 100.4 1012 cm-2 for 20 kGy, which caused from the structural disorder created after γ-radiation. However, they present lowest values at 60 kGy. XRD data was applied to evaluate lattice parameters ‘a’ and ‘c’, unit cell volume (V), X-ray density (dx) and porosity (P) using the following expressions [26]:
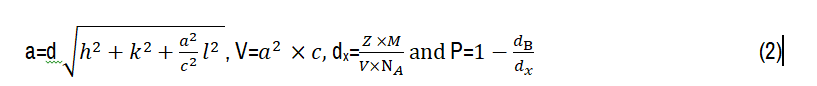
Where; d, (h, k, l), M, Z, NA and dB are respectively: The inter-planar spacing, miller indices, molecular weight=489 gmol-1, atoms number per unit cell, Avogadro number=6.0022*1023 mol-1 and bulk density. Structural parameters were summarized in Tables 1 and 2. γ-radiation provokes a considerable change in lattice parameters ‘a’ and ‘c’, leading to enlarge the unit cell volume (V). Such distance change between copper, indium, tin and sulfur can lead to an expansion of unit cell volume (V). Raimond B. G et al [27] have reported that materials will undoubtedly heat up because of exposing by γ-radiation. Thus, it would lead to cumulative increase of crystal temperature. As result of these investigations, X-ray density was decreased and promotes a gradual increment in the film porosity. This trend is desired for the increase of the void space percentage in the film, which is important to uplift the photocatalytic performances.
Rietveld analysis
MAUD software was performed in order to analyse experimental XRD spectra and evaluate lattice parameters, crystallite size, and microstrain. Figure 4 depicts the Rietveld refinements of non-irradiated and irradiated CITS thin films. Herein, all peaks of CITS materials appear in accordance with those involved in the CIF file. No other feature peaks were found for impurities, proving a complete ordering of atoms in stannite crystal structure. During the fitting process, scale factor, atomic positions, occupancy factors and background coefficients were slightly varied with respecting the experimental XRD spectra. After γ-radiation, it is clearly seen that the peak related to (204) lattice plan depicted at 2θ47.59° was divided after gamma-irradiation on two peaks located at 2θ46.39° and 47.63°. This behavior was observed in annealed CFTS material by F. Ozel et al [28].
Additionally, the peak shown at 2θ56.54 which corresponds to (312) plan was decoupled in two peaks at 2θ55.62° and 56.75° for 40 and 100 kGy. γ-rays with 40, 80 and 100 kGy, lead to appear the small characteristic peaks at 2θ38.44°, 42.24°, 59.53°, 63.86° and 64.84° which assigned to the (202), (114), (224),(321) and (314) lattice plans of stannite structure. These peaks are impeccably matched well with those reported by H. Guan et al [29]. Moreover, all diffraction peaks were shifted as depending on the gamma-doses suggesting a variation of lattice parameters (a) and (c) under the effect of gamma-radiation. We note an increment in the lattice parameters (a) and (c) for gamma radiation up to 40 kGy, which can be attributed to the expansion of the crystal lattice, in fact the unit cell volume V increases after γ-radiations (Table 2). In addition, the microstrain (r.m.s) was increased after irradiation except for 60 kGy-dose, suggesting a large distance between the lattices planes of crystal caused by the stress created from γ-rays (for γ-doses different to 60 kGy). Estimated crystallites size from MAUD software was found nearly to those calculated by Debye-Scherrer formula. As can be understood from Rietveld refinements, exposing to gamma-radiation with 60 kGy enhances the morphology of CITS thin film. Meanwhile, 20-40-80 and 100 kGy deteriorate the crystalline quality of CITS nevertheless no sign of amorphous character.
| g-doses (kGy) | Nc*1012 (cm-2) | V(A3) | dx*10-23 (g/A3) | P (%) | |
|---|---|---|---|---|---|
| 0 | 173 | 5.8 | 314 | 4.13 | 27 |
| 20 | 12-34 | 100-4 | 323 | 4.02 | 29 |
| 40 | 6.94 | 42.3 | 325 | 3.99 | 32 |
| 60 | 1.27 | 3_3 | 327 | 3.97 | 36 |
| 80 | 2.26 | 8.0 | 326 | 3.98 | 34 |
| 100 | 3.08 | 12.6 | 328 | 3.96 | 35 |
Morphological analysis
Figure 5 illustrates the SEM micrographs of non-irradiated and γ-irradiated CITS thin films. It is clearly observed that the surface morphology of CITS material is strongly affected by γ-rays. The micrographs showed a transformation in film morphology from smooth surface to rugged one with the decrease in film thickness. A reveal significant differences in the grain sizes and their shapes for each absorbed dose. Irradiated CITS with 20 and 40 kGy present degraded grains and even broken with irregular sizes. Meanwhile, it can be noticed that the film induced by 60 kGy showed regular features and homogeneous sizes with pyramid morphology. This crystalline shape can change the surface roughness and it gives rise to multireflections that catch the reflected radiation and improve the absorptivity of samples [30]. Markedly, we note the presence of irregular flattened shapes at 80 kGy. Inspection of these images reveals at 100 kGy, the grains are clearly altered and divided into hierarchical rods and lamellar shapes with different dimensions. This result is intended for solar cell applications, which can help in preventing recombination also to improve photocattalytic activity [31]. Figure 6 contains the cross-section images of non-irradiated and γ-irradiated CITS thin film by 60 kGy. It can easily observe that irradiated CITS by 60 kGy exhibits a rugged surface. The film thickness of irradiated CITS by 60 kGy was higher (1.409 μm) than non-irradiated one (0.832 μm), which might be correlated to the increase of crystallite size.
Optical property
Tauc-relation was employed to estimate the energy band gap (Eg) values as presented in the following relation [32]:

Where, A is a parameter depending on transition probability, n=½ for direct band gap, hν (eV) is defined as the incident photon energy and Eg (eV) is the energy band gap. Figure 7 displays the optical band gap of CITS before and after γ-radiation. We notice from the plots that Eg was influenced by high γ-radiation. The same behavior was observed for CoPc [14] and AgSbSe2 materials [33]. Hence, this decrease after γ-radiation may be caused by the defects, which can produce localized state within the band gap that in turn decreases Eg [34]. A.M. Ibrahim et al [16] sayed that the interaction mechanism of γ-radiation with material exhibits an energy transfer to the target atom, it induces a transfer of holes from the valence band to the conduction band with excess number of free electrons and holes that can move in film and enhance its electrical conductivity. Here, Eg was decreased from 1.4 at 0 kGy to 0.95 eV at 20 kGy therefore it was increased up to 1.3 eV at 60 kGy.
Wettability measurement
Controlling the wettability of materials-surface has great practical importance in various areas such as water splitting, photocatalysis, biosensors and flotation [35]. It allows to investigate the difference of energy between the molecules, which exist on the surface and material bulks. The contact angle (θY) of dropped liquid was defined as the consequence of thermodynamic equilibrium of free energy within the solid-liquid-vapor phase. θY was described in 1805 by Thomas Young under the action of three interfacial tensions using equation 4 [36]:

Where γSV,γLV and γSL are respectively correspond to the interfacial tensions with solid/vapor, liquid/vapor and solid/liquid. Figure 8 displays the water contact form on CITS thin films before and after irradiation. It is clearly seen that gamma-radiation leads to decrease θY from 89° for 0 kGy to a minimum value about 43° for 60 kGy. Irradiated CITS thin films revealed a hydrophilic character with θ_Y<90º. In our case, the highest contact angle was obtained for non-irradiated sample, because of its homogeneous and uniform surface compared to irradiated samples. The conversion from hydrophobic to hydrophilic nature related to the modification of surface free energy and surface morphology. The weak adhesive force accompanied with Van der Waals forces between the water and material surfaces can also explain it [37]. The curve evolution clearly indicates that droplet water form has modified from spherical to Elliptical form. It’s worthy to mention that films with lower contact angle (hydrophilic character) showed large surface area between solid and liquid [38]. Thus, hydrophilic surface type supports the physisorption processes that is mandatory for photocatalytic activity [39].
From θY we can explain the wetting of liquids on solid surface with roughness and degree of heterogeneity. Roughness factor R was evaluated by Wenzel's equation, which defined as the area ratio of rough film surface on smooth surface corresponding to it [40]:

Table 3 shows the values of roughness factor of non-irradiated and irradiated CITS thin layers. Inset results suggesting that R does not reflect a coherent tendency with water contact angle. The highest surface roughness value (0.73) corresponds to the CITS film irradiated with 60 kGy accruing the lowest contact angle and highest porosity (~36%). This behavior may be attributed to the regular features and pyramid morphology of grains as observed for CITS irradiated with 60 kGy (Figure 5). These results allow to a very short time reaching surface wetting equilibrium that considered as an advantage in photocatalysis treatment [38].
| g-doses (kGy) | R | γSL(N/m) 10-3 | Wad(N/m) 10-3 | d(mm) | A(mm2) | h(mm) |
|---|---|---|---|---|---|---|
| 0 | 0.02 | 29 | 74 | 1.16 | 1.99 | 0.54 |
| 20 | 0.14 | 31 | 82 | 2.43 | 7.87 | 1.08 |
| 40 | 0.24 | 33 | 90 | 2.57 | 8.10 | 1.00 |
| 60 | 0.73 | 42 | 125 | 3.05 | 8.35 | 0.87 |
| 80 | 0.53 | 39 | 110 | 2.83 | 8.17 | 0.92 |
| 100 | 0.54 | 40 | 111 | 2.92 | 8.69 | 0.95 |
The surface free energy was calculated by employing the state model equation [41]:
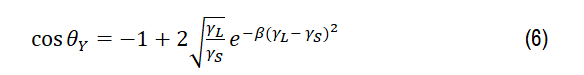
Where,γSV= γS and γLV=γL. A hypothesis was commonly applied which negligees the liquid-vapor adsorption, where β 1.247 10-4 (mJ-2.m4). As the results listed in Table 3, it’s shown that the hydrophilic type dominates by a lower surface free energy for all irradiated films.
On the other hand, Young−Dupré equation describes the effect of wettability on the work adhesion, which mainly used to study the thermodynamic stability of material−liquid interface [40]:
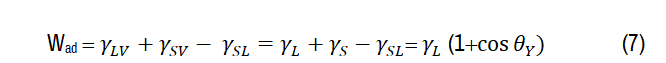
Where, γL= 72.1 mN/m for water
At 60 kGy, irradiated CITS-water interface presents the highest work adhesion (125*10-3 N/m) corresponding to the lowest contact angle (~43°). Hydrophilic surface type increases the adhesion between surface-film and water. It seems that irradiated CITS with 60 kGy is favorable to improve the active groups for hydrogen bond evolution with water, therefore it enhances the wettability (θ<90°) [40]. By comparing the experimental results, it is obvious that γ-ray at 60 kGy leads to increase the surface free energy achieving high adhesive interaction (high Wad) to reach the hydrophilicity character (θ<90°). Therefore, these results are beneficial for using this candidate in photocatalysis and self-cleaning applications [42].
Photocatalytic degradation of RhB solution under xenon light
Based on the relationship between photocatalysis and hydrophilicity, a series of photocatalysis experiments for RhB degradation by irradiated CITS at 60 kGy were made and illustrated in Figure 9. The highest peak intensity depicted at 550 nm is attributed to RhB monomer component. A quick decolourization of RhB occurred in the presence of irradiated CITS with 60 kGy as soon as the first 30 min finished explained by the decrease of peak intensity at 550 nm. Because the photocatalytic activity is commonly believed to occur on solid-surface area, which can present low adsorption value, irradiated CITS with 60 kGy contributes automatically to higher degradation of RhB dye. The above experimental results suggested that irradiated CITS with 60 kGy has a significant photocatalytic activity for RhB under Xenon light irradiation during 120 min.
Stability and recycling tests of photocatalytic degradation
For the economic reasons, it was important to study the recycling repeatability and stability of irradiated CITS by 60 kGy photocatalyst. Figure 10 shows the temporal photodegradation of RhB dye under Xenon illumination during the second, third and fourth cycles. After each cycle that lasted 120 min, irradiated CITS photocatalyst was recovered, washed with bi-distillated water and ethanol, dried in a furnace at 80°C and reused in new RhB solutions under the same conditions. Taking into account the powder loss of photocatalyst during each cycle, the filtration process of RhB solutions was carried out. As can be seen, RhB dye was continuously degraded during the four cycles, meaning that irradiated CITS with 60 kGy is an efficient catalyst with good stability for the remediation of polluted water.
Kinetics of photocatalytic degradation
For qualitatively investigate the photocatalysis activity, the kinetic constants (k) were determined from Langmuir-Hinshelwood model [43],-ln (Ct/C0) was plotted against the time (t). Apparently, the degradation of dye follows the first order kinetics k, as provided in the following equations:
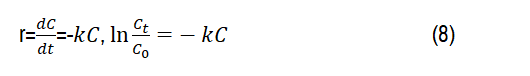
Where, C0 and Ct are respectively the absorbance of the initial dye (t=0) and the absorbance of the dye at any time (t=t). Figure 10b displayed the kinetic spectra of RhB degradations under Xenon irradiation during four cycles. Based on experimental data, kinetic constants k (RhB) of 1st, 2nd, 3rd and 4th cycles are calculated to be 0.018; 0.010; 0.007 and 0.006 min-1,
Photocatalytic degradation efficiency
To examine the capacity of irradiated CITS with 60 kGy for the decolourization of RhB dye, photodegradation rate was calculated using equation 9 [44]:

Figure 11 describies the schematic diagram of photodegradation rate using irradiated CITS under Xenon illumination during four cycles. In our case, photodegradation rate presents 88% after the first cycle, showing a good adsorption capacity of RhB dye. Eventually, the experimental results clearly suggest that RhB dye was decomposed over the second cycle with 70% efficiency. Owing to the stability of irradiated CITS by 60 kGy, photodegradation rate reached 58% after cycle-3. Therefore, RhB dye was continously decolorized with 51% within the fourth cycles, but overall, irradiated CITS at 60 kGy still considered as an efficient and stable photocatalyst for water purification.
In summary, this paper covers results concerning the effect of γ-rays with different doses (20-40-60-80 and 100 kGy) on chalcogenide Cu2InSnS4 (CITS) thin films. XRD analysis demonstrates that CITS material keeps its stannite structure after irradiation followed by little deteriorationn in crystallinity. The samples have different surface morphologies with general decrease of grain size, which may be due to the strong capacity of γ-radiation to penetrate through the targeted to reduce crystallite size into smaller ones. Noticeable optical changes have been observed in CITS thin layers during the irradiation process. After γ-ray, Eg was decreased to a minimum value of about 0.95 eV. In addition, wettability experiments showed that γ-radiation transforms the surface nature from hydrophobic (θ>90°) to hydrophilic (θ<90°) and pave the way for possible use of irradiated CITS with 60 kGy in various sensitivity applications such as self-cleaning and photocatalysis applications. Our findings confirm that irradiated CITS at 60 kGy has received relatively little attention in photocatalysis application. The highest photodegradation rate of RhB under Xenon illumination after 120 min was equal to 88%. Furthermore, the fourth repeated cycles of RhB degradation can make irradiated CITS kind film as promising alternative in up-scaled photocatalytic treatment for advanced water re-use.
The Authors are highly indebted to the Deanship of the Scientific Research (DSR), Umm Al-Qura University for the financial support through the project number 19-SCI- 1-01-0044 entitled: Synthesis, development and systematic study of the performances of a novel Photovoltaic Thermoelectric nanocomposite-based hybrid solar cells.
Journal of Material Sciences & Engineering received 3677 citations as per Google Scholar report