Research Article - (2022) Volume 0, Issue 0
Received: 15-Aug-2022, Manuscript No. JBL-22-71866;
Editor assigned: 17-Aug-2022, Pre QC No. JBL-22-71866;
Reviewed: 31-Aug-2022, QC No. JBL-22-71866;
Revised: 07-Sep-2022, Manuscript No. JBL-22-71866;
Published:
14-Sep-2022
, DOI: 10.37421/2165-7831.2022.12.012
Citation: Hakseven, Musluh, Özhan Çetindağ, Gökhan Avşar and Rıza Deryol, et al. "Evaluation of Preoperative Fibrinogen/Albumin Ratio and Carcinoembryonic Antigen: A New Prognostic Marker in Gastric Cancer". J Blood Lymph 11(2022): 012
Copyright: © 2022 Hakseven M, et al. This is an open-access article distributed under the terms of the creative commons attribution license which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Introduction: Gastric Cancer (GC) is one of the most common cancers that can result in death. Markers are needed to detect gastric cancer early and manage treatment. We aimed to reveal the relationship between Carcinoembryonic Antigen (CEA) level and Fibrinogen-Albumin Ratio (FAR) and prognosis in gastric cancer, as well as to examine the relationship of these values with the number of metastatic lymph nodes and TNM stage.
Materials and methods: The data of 805 consecutive gastrectomy patients were analyzed retrospectively. A total of 461 patients were included. The optimal cut- off values of CEA and FAR were 2.43 ng/mL and 1.26, respectively. Patients were stratified into three groups based on this cutoff value: CEA-FAR=0 (CEA<2.43 ng/mL and FAR<1.26), CEA-FAR=1 (CEA ≥ 2.43 ng/mL or FAR ≥ 1.26), and CEA-FAR=2 (CEA ≥ 2.43 ng/mL and FAR ≥ 00201.26).
Results: There was a significant relationship between high CEA and stage (p=0.040), N status (p=0.017), and lymph node metastasis (p=0.004), and also there was a significant correlation between high FAR value and grade (p=0.003), stage (p<0.001), T status (p<0.001), N status (p<0.001) and metastatic lymph node count (p<0.001). Overall and disease-free survival were significantly different between the three CEA-FAR groups.
Conclusion: We believe that pre-operative FAR and CEA values are independent predictors of survival. FAR and CEA are potential prognostic indicators for resectable gastric cancer due to their easy access and low cost. Considering survival and prognosis in patients with very high preoperative CEA and FAR values, neoadjuvant chemotherapy should also be considered.
Gastric cancer • Prognosis • Fibrinogen/albumin ratio • Survival • New tumor marker
GC: Gastric Cancer; CEA: Carcinoembryonic Antigen; FAR: Fibrinogen/ Albumin Ratio; CA19-9: Carbohydrate Antigen 19-9; CT: Computed Tomography; MRI: Magnetic Resonance Imaging; ASA: The American Society of Anesthesiologists.
Gastric Cancer (GC) is one of the most commonly encountered cancers and metastases are not infrequent at diagnosis. Markers are needed for early detection of the disease and predicting its prognosis. There is no standard predictor other than TNM classification for prognosis. It is known that patients with the same stage according to TNM classification show survival differences, so it is thought that there are other factors affecting prognosis and survival.
Fibrinogen, one of the glycoproteins synthesized by the liver, increases in response to inflammation or by activation of the coagulation system. Fibrinogen is an acute-phase reactant, and malignant tumors can cause abnormal coagulation functions and increases fibrinogen. In the literature, increased plasma fibrinogen level has been found to be associated with gastric cancer and poor prognosis [1]. High fibrinogen levels and decreased albumin levels are associated with systemic inflammation.
Malnutrition is a common clinical manifestation of gastrointestinal cancers and is also a prognostic marker of cancer. Albumin blood level measurement is often one of the ways to evaluate nutritional status, and hypoalbuminemia is associated with poor prognosis in many cancers such as lung, stomach and colon cancer [2].
There is a need for rapid and inexpensive markers that can be more easily evaluated in gastric cancer. In clinical practice, Carcinoembryonic Antigen (CEA) and Carbohydrate Antigen (CA) 19-9 are the most widely used markers for early detection and follow-up of gastric cancer. These markers have been confirmed to be associated with prognosis and postoperative recurrence [3]. Tumor markers can be helpful in diagnosis, treatment follow-up, and prognosis prediction in gastric cancer patients. CEA is a common tumor marker for gastrointestinal cancers and has come to the fore in gastric cancer. High CEA is a risk factor for poor prognosis in gastric cancer [4].
In this study; we aimed to reveal the relationship between CEA level and Fibrinogen-Albumin Ratio (FAR) and prognosis in gastric cancer, as well as to examine the relationship of these values with the number of metastatic lymph nodes and TNM stage detected after pathological examination. Investigation and standardization of these factors will provide an advantage in terms of prognosis.
Patients
Patients who underwent curative resection for gastric cancer in Ankara University, Department of Surgical Oncology between January 2008 and January 2018 were analyzed retrospectively. Patients whose hospital data were completely recorded, who did not receive neoadjuvant chemoradiotherapy, who did not have a history of coagulation disorder and anticoagulant use, and who did not have metastases during imaging and surgery were included in the study. Patients who underwent palliative surgery, had a hematological disease, cancer of other organs, and patients with liver and kidney disease were excluded from the study. This study was carried out in accordance with the Declaration of Helsinki with the approval of the ethics committee of Ankara University Faculty of Medicine.
Detailed clinicopathological and demographic data, including patient age, sex, tumor size and location, histological grade, tumor stage (TNM classification, AJCC 8th Edition), extent of gastrectomy, early postoperative outcomes, preoperative peripheral blood count values, albumin, fibrinogen, and tumor markers such as CEA were retrospectively scanned from the hospital database and recorded. Fibrinogen to Albumin Ratio (FAR) was calculated by dividing the fibrinogen level (g/l) by the serum albumin level (g/dl) [5].
Patient follow-up included hematological and biochemical tests, tumor markers, Computed Tomography (CT) or Magnetic Resonance Imaging (MRI), and endoscopic evaluation. CT/MRI was performed every six months for the first two years and then annually, while endoscopy was performed annually. Survival time was calculated from the date of diagnosis to the date of death or the last follow-up.
The data of 805 consecutive patients who underwent gastrectomy for gastric cancer in our clinic were reviewed retrospectively. 91 patients were excluded because of a history of neoadjuvant chemotherapy or neoadjuvant chemoradiotherapy. 74 patients were found to have coagulation disorder or anticoagulant use and were excluded from the study. 179 patients were excluded from the study due to lack of records or unavailability of survival information.
Statistical analysis
Optimal cut-off points for CEA and FAR values were determined by ROC (Receiver Operating Characteristic) analysis for overall survival and disease-free survival. According to these cut-off points, patients were divided into two groups as high (1) and low (0) for CEA and FAR. In addition, patients were divided into three groups according to their CEA-FAR values (6). CEA-FAR=0 (CEA<2.4 ng/mL and FAR<1.26), CEA-FAR=1 (CEA ≥ 2.4 ng/mL or FAR ≥ 1.26) and CEA-FAR=2 (CEA ≥ 2.4 ng/mL and FAR ≥ 1.26). Chi-square and Fisher's exact test were used in categorical data analysis. Survival analysis was performed according to the Kaplan-Meier method. Multivariate Cox analysis was used to determine the factors affecting overall survival and disease-free survival. Multivariate analysis was conducted with variables that were statistically significant in univariate analysis (Table 1).
| Overall survival | Disease-free survival | ||
|---|---|---|---|
| Scoring system | Score | Scoring system | Score |
| CEA (ng/ml) | CEA (ng/ml) | ||
| <2.43 | 0 | <2.4 | 0 |
| ≥ 2.43 | 1 | ≥ 2.4 | 1 |
| FAR | FAR | ||
| <1.26 | 0 | <1.26 | 0 |
| ≥ 1.26 | 1 | ≥ 1.26 | 1 |
| CEA-FAR | CEA-FAR | ||
| CEA<2.43 and FAR <1.26 | 0 | CEA<2.4 and FAR <1.26 | 0 |
| CEA ≥ 2.43 or FAR ≥ 1.26 | 1 | CEA≥2.4 or FAR ≥1.26 | 1 |
| CEA ≥ 2.43 and FAR ≥ 1.26 | 2 | CEA≥2.4 and FAR ≥1.26 | 2 |
All statistical analysis was performed in the SPSS 26.0 package program and p<0.05 was considered statistically significant.
The study was conducted on 461 patients who underwent curative surgery for gastric cancer. 302 (65.5%) of the patients were male and 159 (35.5%) were female. The median age of the patients was 63 years (19-96).
Clinicopathological parameters of the patients are summarized in Table 2.
| Parameters | Patients n (%) | CEA-FAR | CEA-FAR | CEA-FAR | χ2 | p-value |
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | ||||
| Age | 1.494 | 0.474 | ||||
| <63 | 227(49.2%) | 56 | 99 | 72 | ||
| ≥ 63 | 234(50.8%) | 50 | 115 | 69 | ||
| Gender | 1.589 | 0.452 | ||||
| Male | 302(65.5%) | 66 | 138 | 98 | ||
| Female | 159(35.5%) | 40 | 76 | 43 | ||
| Grade | ||||||
| 1 | 41(8.9%) | 13 | 20 | 8 | 8.07 | 0.089 |
| 2 | 145(31.5) | 41 | 61 | 43 | ||
| 3 | 275(59.7) | 52 | 133 | 90 | ||
| Stage | 32.471 | <0.001 | ||||
| I | 13(2.8%) | 7 | 5 | 1 | ||
| II | 175(38.0%) | 59 | 77 | 39 | ||
| III | 273(59.2%) | 40 | 133 | 100 | ||
| T Status | 23.953 | <0.001 | ||||
| 1 | 21(4.5%) | 11 | 9 | 1 | ||
| 2 | 32(7.0%) | 14 | 11 | 7 | ||
| 3 | 286(62.0%) | 60 | 137 | 89 | ||
| 4 | 122(26.5%) | 21 | 57 | 44 | ||
| N Status | 36.932 | <0.001 | ||||
| 1 | 113(24.5%) | 45 | 44 | 24 | ||
| 2 | 131(28.4) | 33 | 67 | 31 | ||
| 3 | 217(47.1%) | 28 | 103 | 86 | ||
| No of metastatic lymph nodes | 32.069 | <0.001 | ||||
| <6 | 70 | 98 | 42 | |||
| ≥ 6 | 36 | 116 | 99 | |||
| Localization | 5.248 | 0.512 | ||||
| Antrum | 156(33.8%) | 37 | 69 | 50 | ||
| Corpus | 229(49.7%) | 56 | 109 | 64 | ||
| Diffuse | 11(2.4%) | 0 | 7 | 4 | ||
| Cardia | 65(14.1) | 13 | 29 | 23 | ||
| ASA | 4.945 | 0.293 | ||||
| 1 | 220(47.7%) | 55 | 100 | 65 | ||
| 2 | 187(40.6%) | 45 | 85 | 57 | ||
| 3 | 54(11.7%) | 6 | 29 | 19 |
The cut-off value for CEA values based on survival groups (high=1, low=0) determined by median overall survival (MD=11, 10-153 months) was 2.43 ng/mL (Area under the curve, AUC=0.615, 95% CI: 0.562- 0.668, p<0.001); The cut-off value obtained for the FAR value was 1.26 (AUC=0.749, 95% CI: 0.699-0.798, p<0.001). According to the cut-off values, for CEA and FAR values patients were divided into 2 groups; as high CEA (≥ 2.43 ng/mL, n=214) and low CEA (<2.43 ng/mL, n=247); and also as high FAR (≥ 1.26, n=282) and low FAR (<1.26, n=179).
The cut-off value for CEA values based on the survival groups (high=1, low=0) determined by the median value of disease-free survival (MD=9, 0-148 months) was 2.4 ng/mL (AUC=0.628, 95% CI: 0.574-0.682, p<0.001); The cut-off value obtained for the FAR value was 1.26 (AUC=0.773, 95% CI: 0.724-0.821, p<0.001). According to the cut-off values, for CEA and FAR values patients were divided into 2 groups; as high CEA (≥ 2.4 ng/mL, n=214) and low CEA (<2.4 ng/mL, n=247); and also as high FAR (≥ 1.26, n=282) and low FAR (<1.26, n=179).
CEA and FAR cut-off values based on total survival and disease-free survival are very close to each other, and the number of patients in the groups formed according to these values is equal.
When the relationship between CEA and FAR values and clinicopathological values of the patients was examined, there was a significant relationship between high CEA and stage (p=0.040), N status (p=0.017), and lymph node metastasis (p=0.004). The CEA value of the patients in stage 3 was significantly higher than the patients in stage 1 and stage 2; The CEA value of patients with N3 was significantly higher than those with N1 and N2. According to the number of metastatic lymph nodes, it was seen that the CEA value of patients with metastatic lymph node number below 6 is significantly lower, and the CEA value of patients with 6 and above is significantly higher. There was no significant relationship between the CEA value and ASA, age, gender, grade, T status, and tumor localization of the patients (p>0.05).
There was a significant correlation between high FAR value and grade (p=0.003), stage (p<0.001), T status (p<0.001), N status (p<0.001) and metastatic lymph node count (p<0.001). The FAR value was significantly lower in grade 1 and significantly higher in grade 3; significantly lower in stage 1 and stage 2, and significantly higher in stage 3; significantly lower in T1 and T2, significantly higher in T4; significantly lower in N1, significantly higher in N3; It was seen that the number of metastatic lymph nodes was significantly lower when below 6, and it was significantly higher in patients with 6 and above. There was no significant correlation between the FAR value and ASA, age, gender, and tumor localization of the patients (p>0.05).
The relationship between CEA-FAR values and clinicopathological values of GC patients is presented in Table 3. There was a significant correlation between high CEA-FAR value and stage (p<0.001), T status (p<0.001), N status (p<0.001) and lymph node metastasis (p<0.001). While both CEA and FAR values of patients in stage 1 are both significantly below the cut-off point, in stages 2 and 3, one or both of the CEA-FAR values are significantly above the cut-off score. While both CEA and FAR values are significantly lower in N1 patients, one or both CEA-FAR values are significantly higher in N3. CEA and FAR values are both significantly lower in T1 and T2. One or both of the CEA and FAR values are significantly higher in patients with metastatic lymph node count greater than 6. Since the number of patients in the groups formed from the cut-off values of CEA, FAR and CEA-FAR for total survival and disease-free survival is equal, the relationship of CEA, FAR and CEA-FAR with clinicopathological variables does not change for both overall survival and disease-free survival (Table 3).
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | Coeff. (bi) | HR(exp(bi)) | 95% CI | p-value | Coeff. (bi) | HR(exp(bi)) | 95% CI | p-value |
| Age | 0.002 | 1.002 | (0.933-1.010) | 0.729 | ||||
| Gender | 0.034 | 1.034 | (0.919-1.164) | 0.579 | ||||
| BMI | 0.055 | 1.056 | (0.997-1.120) | 0.065 | ||||
| ASA | ||||||||
| I | 0 | -1 | 0.003 | 0.006 | ||||
| II | 0.031 | 1.031 | (0.810-1.313) | 0.802 | -0.059 | 0.943 | (0.737-1.206) | 0.639 |
| III | 0.576 | 1.779 | (1.268-2.497) | 0.001 | 0.497 | 1.644 | (1.165-2.318) | 0.005 |
| Grade | ||||||||
| I | 0 | -1 | <0.001 | 0.001 | ||||
| II | 0.511 | 1.668 | (0.990-2.809) | 0.055 | 0.379 | 1.461 | (0.864-2.470) | 0.157 |
| III | 0.98 | 2.664 | (1.623-4.373) | <0.001 | 0.756 | 2.129 | (1.286-3.525) | 0.003 |
| Stage | ||||||||
| I | 0 | -1 | <0.001 | 0.151 | ||||
| II | 1.656 | 5.24 | (1.293-21.231) | 0.020 | 0.916 | 2.499 | (0.438-14.447) | 0.303 |
| III | 2.035 | 7.65 | (1.889-30.818) | 0.004 | 0.557 | 1.746 | (0.285-10.697) | 0.547 |
| FAR | 0.149 | 1.161 | (1.111-1.214) | <0.001 | 0.126 | 1.134 | (1.072-1.200) | <0.000 |
| CEA (ng/mL) | 0.001 | 1.001 | (1.000-1.001) | 0.001 | 0.001 | 1.001 | (1.000-1.001) | 0.004 |
| T status | ||||||||
| 1 | 0 | -1 | <0.001 | 0.513 | ||||
| 2 | 0.546 | 1.726 | (0.670-4.449) | 0.258 | 0.13 | 1.139 | (0.372-3.484) | 0.82 |
| 3 | 1.171 | 3.225 | (1.431-7.270) | 0.005 | 0.173 | 1.189 | (0.430-3.291) | 0.739 |
| 4 | 1.381 | 3.978 | (1.741-9.092) | 0.001 | 0.401 | 1.494 | (0.512-4.356) | 0.462 |
| N status | ||||||||
| 1 | 0 | -1 | <0.001 | 0.006 | ||||
| 2 | 0.038 | 1.039 | (0.744-1.451) | 0.821 | -0.483 | 0.617 | (0.435-0.877) | 0.007 |
| 3 | 0.525 | 1.69 | (1.262-2.263) | <0.001 | 0.018 | 1.018 | (0.672-1.542) | 0.933 |
| No of metastatic lymph nodes | 0.6 | 1.822 | (1.443-2.301) | <0.001 | -2.96 | 0.052 | (0.019-0.141) | <0.001 |
Survival analyses for CEA and FAR
According to Kaplan-Meier analysis and Log-rank tests, the overall survival time of the low CEA group was 66.68 ± 4.95 (CI: 57.78-75.58) significantly higher than the overall survival time of the high CEA group, 41.50 ± 4.29 (CI: 33.08-49.92), p<0.001. According to Kaplan-Meier analysis and Log-rank tests, the disease-free survival time of the low CEA group was 62.14 ± 4.40 (CI: 53.5-70.77) significantly higher than the disease-free survival time of the high CEA group, 34.09 ± 3.87 (CI: 26.51-41.68), p<0.001 (Figures 1A and 1B).
Similarly, the overall survival time of the low FAR group was 87.21 ± 5.36 (CI: 76.70-97.71) significantly higher than the overall survival time of the high FAR group, 32.19 ± 3.17 (CI: 25.96-38.41), p<0.001, and the disease-free survival time of the low FAR group was 83.66 ± 5.23 (CI: 73.40-93.93) significantly higher than the disease-free survival time of the high FAR group of 16.74 ± 1.59 (CI: 13.16-19.86), p<0.001 (Figures 2A and 2B).
Survival analyses for CEA-FAR
In this study, there was a significant difference in overall survival between the three CEA-FAR groups (CEA-FAR=0, 1 and 2: 98.39 ± 6.69 (CI: 85.29-111.50), 52.12 ± 4.62 (CI: 43.05-61.18), 24.76 ± 3.97 (CI: 16.97-32.55); p<0.001. Kaplan-Meier analysis was performed for overall survival based on CEA-FAR by stage, N status, T status, and metastatic lymph node count.
CEA-FAR was found to have a predictive value for pathological stage 3 (CEA-FAR=0, 1 and 2: 90.71 ± 7.01 (CI: 76.95-104.46), 41.67 ± 4.12 (CI: 33.60-49.74), 21.43 ± 3.74 (CI: 14.08-28.79); p<0.001.
CEA-FAR value was found to predict overall survival of N2 (CEA-FAR=0, 1 and 2: 97.41 ± 6.84 (CI: 84.04-110.84), 68.08 ± 6.23 (CI: 55.88-80.30), 59.92 ± 10.37 (CI: 39.60-80.24); p=0.001 and N3 (CEA-FAR=0, 1 and 2: 106.93 ± 7.11 (CI: 92.99-120.86), 55.13 ± 5.41 (CI: 44.51-65.75), 26.82 ± 4.78 (CI: 17.46-36.19); p<0.001 patients.
CEA-FAR value was found to predict overall survival in T3 (CEA- FAR=0, 1 and 2: 73.64 ± 5.8 (CI: 62.26-85.07), 39.77 ± 3.88 (CI: 32.16- 47.38), 26.09 ± 4.56 (CI: 17.15-35.03); p<0.001 and T4 (CEA-FAR=0, 1 and 2: 116.28 ± 6.89 (CI: 102.78-129.78), 81.37 ± 7.16 (CI: 67.32-95.42), 55.88 ± 9.4 (CI: 37.42-74.34); p<0.001 patients.
Overall survival time was significantly longer in patients with metastatic lymph node count below 6 and CEA-FAR value together below the cut- off score, (CEA-FAR=0, 1 and 2: 67.58 ± 5.1 (CI: 57.76-77.41), 52.29 ± 4.73 (CI: 43.02-61.57), 50.96 ± 8.61 (CI: 34.07-67.85); p=0.024). Overall survival time of patients with 6 or more metastatic lymph nodes differed significantly between all three CEA-FAR groups (CEA-FAR=0, 1 and 2: 96.86 ± 7.01 (CI: 82.96-110.77), 49.39 ± 4.97 (CI: 39.64-59.13), 22.33 ± 3.95 (CI: 14.58-30.07); p<0.001.
Disease-free survival was significantly different between the three CEA-FAR groups (CEA-FAR=0, 1 and 2: 96.80 ± 6.52 (CI: 84.00-109.58), 44.97 ± 4.28 (CI: 36.58-53.36), 11.89 ± 1.81 (CI: 8.44-15.53); p<0.001) (Figures 3A,3B and 4A-4F).
Figure 4. A. Overall survival according to CEA-FAR classification for stage 3; B.
Overall survival according to CEA-FAR classification for N2; C. Overall survival
according to CEA-FAR classification for N3; D. Overall survival according to CEA-
FAR classification for T3; E. Overall survival according to CEA-FAR classification
for T4; F. Overall survival according to CEA-FAR classification for more than 6
metastatic lymph nodes 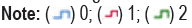
In stage 1 and stage 2, when both CEA-FAR values are below the cut- off value, disease-free survival increases significantly, while a high CEA- FAR value for stage 3 patients significantly reduces disease-free survival (CEA-FAR=0, 1 and 2: 57.41 ± 4.71 (CI: 48.17-66.66), 51.66 ± 5.13 (CI: 41.56-61.76), 33.40 ± 4.37 (CI: 24.85-41.98) p<0.001; CEA-FAR=0, 1 and 2: 82.614 ± 7.40 (CI: 68.09-97.13), 36.28 ± 4.14 (CI: 28.26-44.39), 14.89 ± 2.24 (CI: 10.52-19.28); p<0.001.
CEA-FAR value was found to predict disease-free survival of N2 (CEA- FAR=0, 1 and 2: 86.65 ± 7.19 (CI: 72.54-100.76), 58.25 ± 6.26 (CI: 45.97- 70.52), 37.19 ± 4.94 (CI: 27.52-46.88); p<0.001 and N3 (CEA-FAR=0, 1 and 2: 99.08 ± 7.79 (CI: 83.80-114.35), 49.72 ± 5.55 (CI: 38.23-60.61), 18.29 ± 2.74 (CI: 12.92-23.67); p<0.001 patients. CEA-FAR value was found to predict disease-free survival in T3 (CEA-FAR=0, 1 and 2: 61.67 ± 5.58 (CI: 50.73-72.61), 33.24 ± 3.66 (CI: 26.07-40.41), 15.73 ± 2.23 (CI: 11.30- 20.16); p<0.001 and T4 (CEA-FAR=0, 1 and 2: 109.89 ± 7.32 (CI: 95.54- 124.25), 73.34 ± 7.46 (CI: 58.71-87.96), 38.24 ± 4.6 (CI: 29.22-47.26); p<0.001 patients. CEA-FAR value significantly changes disease-free survival in patients with 6 or more metastatic lymph nodes. (CEA-FAR=0, 1 and 2: 88.21 ± 7.57 (CI: 73.37-103.044), 43.99 ± 5.02 (CI: 34.16-53.84), 15.20 ± 2.29 (CI: 10.72-19.69); p<0.001 (Figures 5A-5F).
Figure 5. A. Disease free survival according to CEA-FAR classification for stage
3; B. Disease free survival according to CEA-FAR classification for N2; C. Disease
free survival according to CEA-FAR classification for N3; D. Disease free survival
according to CEA-FAR classification for T3; E. Disease free survival according
to CEA-FAR classification for T4; F. Disease free survival according to CEA-FAR
classification for more than 6 metastatic lymph nodes. 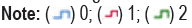
Univariate and multivariate survival analyses
Univariate and multivariate analysis were used to identify clinicopathological variables predicting overall survival and disease-free survival. According to univariate analysis results, ASA (p=0.003), grade (p<0.001), stage (p<0.001), FAR (p<0.001), CEA (ng/ml) (p=0.001), T status (p<0.001), N status (p<0.001), Lymph node metastasis (p<0.001) predicted the overall survival time of patients significantly. The overall survival time of the patients in the ASA 3 group was significantly lower than the patients in the ASA 1 and 2 groups (p=0.001). The overall survival time of the patients in the grade 3 group was significantly lower than the patients in the grade 1 and 2 groups (p<0.001). The overall survival of patients in stage 1 was significantly higher than stage 2 and stage 3 (p=0.020, p=0.004). The overall survival time of patients with T1 and T2 was significantly higher than patients with T3 and T4 (p=0.005, p=0.001). The overall survival time of patients with N1 was significantly higher than patients with N3 (p<0.001). The overall survival time of those with 6 or more metastatic lymph nodes was significantly lower than those with less than 6 (p<0.001). As a result of univariate analysis, the multivariate regression model in which the variables predicting overall survival together predicted overall survival was found to be significant (χ2=147.861, p<0.001). According to multivariate analysis results, ASA (p=0.006), grade (p=0.001), FAR (p<0.001), CEA (ng/ ml) (p=0.004), N status (p=0.006) and number of metastatic lymph nodes (p<0.001) together significantly predicted the overall survival time of the patients.
According to univariate analysis results, BMI (p=0.024), ASA (p=0.005), grade (p<0.001), stage (p<0.001), FAR (p<0.001), CEA (ng/ml) (p=0.002), T status (p<0.001), N status (p<0.001) and metastatic lymph node count (p<0.001) significantly predict disease-free survival of patients (Table 4). The disease-free survival time of the patients in the ASA 3 group was significantly lower than the patients in the ASA I and II groups (p=0.001). Disease-free survival of patients in grade 1 was significantly higher than grade 2 and grade 3 (p=0.034, p=0.001). The disease-free survival time of patients in stage 3 was significantly lower than that of patients in stages 1 and 2 (p<0.009). Disease-free survival time of patients with T1 and T2 was significantly higher than patients with T3 and T4 (p=0.015, p=0.003). The disease-free survival time of patients with N1 was significantly higher than patients with N3 (p<0.001). Disease-free survival of patients with 6 or more metastatic lymph nodes was significantly lower than patients with less than 6 (p<0.001). As a result of univariate analysis, the multivariate regression model in which the variables predicting disease-free survival together predicted disease-free survival was found to be significant (χ2=149,502, p<0.001). According to multivariate analysis results, BMI (p=0.044), ASA (p=0.021), grade (p=0.001), FAR (p<0.001), CEA (ng/ml) (p=0.011), N status and (p=0.004), Lymph node metastasis (p<0.001) predicted disease- free survival time of patients together significantly (Table 4).
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | Coeff. (bi) | HR(exp(bi)) | 95% CI | p-value | Coeff. (bi) | HR(exp(bi)) | 95% CI | p-value |
| Age | 0.002 | 1.002 | (0.993-1.010) | 0.679 | ||||
| Gender | 0.033 | 1.034 | (0.920-1.116) | 0.579 | ||||
| BMI | 0.065 | 1.067 | (1.009-1.130) | 0.024 | 0.061 | 1.063 | (1.002-1.128) | 0.44 |
| ASA | ||||||||
| I | 0 | -1 | 0.005 | 0.021 | ||||
| II | 0.116 | 1.123 | (0.866-1.422) | 0.337 | 0.014 | 1.014 | (0.795-1.294) | 0.911 |
| III | 0.564 | 1.758 | (1.254-2.466) | 0.001 | 0.467 | 1.596 | (1.130-2.254) | 0.008 |
| Grade | ||||||||
| I | 0 | -1 | <0.001 | 0.001 | ||||
| II | 0.576 | 1.779 | (1.043-3.035) | 0.034 | 0.485 | 1.624 | (0.948-2.783) | 0.078 |
| III | 1.096 | 2.993 | (1.799-4.978) | <0.001 | 0.912 | 2.49 | (1.479-4.192) | 0.001 |
| Stage | ||||||||
| I | 0 | -1 | <0.001 | 0.526 | ||||
| II | 0.857 | 2.355 | (0.868-6.395) | 0.093 | 0.166 | 1.18 | (0.280-4.965) | 0.821 |
| III | 1.326 | 3.766 | (1.399-10.132) | 0.009 | -0.077 | 0.926 | (0.202-4.274) | 0.921 |
| FAR | 0.149 | 1.161 | (1.113-1.211) | <0.001 | 0.127 | 1.135 | (1.074-1.199) | <0.000 |
| CEA (ng/mL) | 0.001 | 1.001 | (1.000-1.001) | 0.002 | 0.001 | 1.001 | (1.000-1.001) | 0.011 |
| T status | ||||||||
| 1 | 0 | -1 | <0.001 | 0.684 | ||||
| 2 | 0.21 | 1.233 | (0.523-2.909) | 0.632 | 0.13 | 1.139 | (0.372-3.493) | 0.82 |
| 3 | 0.88 | 2.412 | (1.189-4.890) | 0.015 | 0.143 | 1.154 | (0.416-3.201) | 0.783 |
| 4 | 1.083 | 2.953 | (1.433-6.084) | 0.003 | 0.324 | 1.383 | (0.474-4.035) | 0.553 |
| N status | ||||||||
| 1 | 0 | -1 | <0.001 | 0.004 | ||||
| 2 | 0.005 | 1.005 | (0.722-1.399) | 0.977 | -0.471 | 0.624 | (0.437-0.892) | 0.010 |
| 3 | 0.56 | 1.75 | (1.314-2.332) | <0.001 | 0.081 | 1.084 | (0.716-1.640) | 0.703 |
| No of metastatic lymph nodes | 0.632 | 1.881 | (1.494-2.369) | <0.001 | -2.404 | 0.09 | (0.042-0.195) | <0.001 |
Gastric cancer is frequently encountered as an advanced disease because it is not detected in the early stages and is not easily detected. Despite surgical and medical advances, it still continues to have a poor prognosis. Various markers are used to predict prognosis and manage treatment [6]. CEA is a serum tumor marker that has been used for a long time in the diagnosis and follow-up of gastrointestinal malignancies, and its use as a prognostic predictor has been frequently reported [7,8]. CEA has an important place in the diagnosis of gastric cancer and in the follow-up of treatment, but its diagnostic efficiency is not high enough and may not be an ideal marker due to its relatively low sensitivity and specificity [6,7,9].
Fibrinogen is a proinflammatory protein that plays an important role in the inflammatory response and tumor progression; It is associated with the clinicopathological features and prognosis of many tumors such as breast, lung, prostate and gastrointestinal cancers [10]. Hyperfibrinogenemia is significantly associated with tumor growth, advanced cancer stage, lymph node metastasis and poor prognosis in GC patients [1,11]. It has been found that plasma fibrinogen levels are significantly higher in cancer patients and that fibrinogen levels increase during recurrence or metastasis [12].
Albumin is a negative acute-phase protein that is used as a nutritional marker and decreases with surgical stress and capillary leakage. Measurement of albumin value is frequently used in clinical practice to evaluate nutritional status. Low albumin indicates malnutrition. Malnutrition may lead to suppression of immune function in patients with malignancy, increasing the risk of postoperative complications and poor prognosis [6]. Preoperative albumin level has been reported to be a predictive factor for postoperative recovery and long-term survival in GC patients, and it has been predicted that postoperative reduction of serum albumin level may be a predictive factor for short-term complications in GC patients [13,14].
It is known that high fibrinogen and low albumin levels are separately associated with poor prognosis in cancer patients. We think that the use of FAR will give more accurate results to examine the simultaneous effect of both high fibrinogen and low albumin levels on prognosis. It has been reported in previous studies that high FAR values may be associated with poor prognosis in different cancer types [15-17].
In this study, preoperative CEA elevation was found to be associated with advanced TNM stage and increased metastatic lymph node count. In a comprehensive study, similar to our study, it was shown that pre-operative high CEA level can predict stage 3 patients rather than stage 1 and 2 and is associated with poor prognosis [18].
In the current study, the FAR value also predicts the tumor grade, unlike the CEA value. Studies indicate that the prognostic value of FAR is stronger when compared to that of fibrinogen or albumin alone [19]. In the literature, high preoperative FAR value has been found to be associated with poor overall survival in gastric cancer patients undergoing surgery, independent of pathological stage [20]. In the current study, it was observed that preoperatively elevated FAR value was associated with advanced cancer stage, enlarged tumor size, increased number of metastatic lymph nodes, and poor survival.
There is a significant relationship between high CEA-FAR value and TNM stage. In stage 1 patients, both CEA and FAR values are significantly below the cut-off point, and in stage 2 and 3, one or both of the CEA-FAR values are significantly above the cut-off score (If one or both of these are high, the patient is in stage 2 or 3, if both are low, the patient is in stage 1). While the CEA and FAR values of patients with N1 are both significantly low, one or both of the CEA-FAR values are significant in N3 (If one or both of the patient's CEA or FAR values are above the cut-off score, the patient is in the N3 class; if both are low, the patient is in the N1 class). CEA and FAR values together are significantly low in T1 and T2 (If the two values together are below the cut-off points, it is in the T1 or T2 class). Patients with more than 6 metastatic lymph nodes have significantly higher one or both of CEA and FAR.
Previous studies have found that CEA-FAR is a more effective prognostic marker than CEA or FAR alone in gastric cancer patients who have undergone curative gastrostomy. The current study shows that CEA-FAR concomitant elevation was associated with advanced cancer stage, large tumor size, increased metastatic lymph node count, and poor survival, while low CEA-FAR was found to be associated with lower cancer stage, small tumor size, reduced metastatic lymph node count, and better survival. In agreement with the literature, also our study shows that increased CEA-FAR is associated with tumor volume and tumor progression and is a prognostic factor for gastric cancer.
In univariate and multivariate Cox analysis, variables such as ASA, grade, FAR, CEA, N status, lymph node metastasis can significantly predict disease-free survival and overall survival.
The FAR values were frequently studied as a prognostic factor in recent studies, and especially in studies conducted for gastric cancer; similar results to the current study were obtained. In a subsequent study, a higher FAR value in gastric cancer patients with operable tumors was associated with larger tumor size, poor differentiation, greater metastatic lymph node count, and worse TNM stage. The same study found that higher CEA was associated with increased age, larger tumor size, greater number of metastatic lymph nodes, and worse TNM stage. In this study, we see that CEA and FAR values were associated with TNM stage and the number of metastatic lymph nodes. Therefore, this study reveals that preoperative CEA and FAR values are independent prognostic factors and, when used together, can give an idea about TNM stage and metastatic lymph node number.
In our study, it is seen that the elevation of CEA and FAR predicts disease stage, disease-free survival and overall survival. In the detailed evaluation, it is understood that the elevation of either of the CEA and FAR values, or both, is associated with poor prognosis and advanced disease. In a detailed statistical study with the calculated FAR value, it is seen that the high values of FAR may be more valuable than the high values of CEA, which is a tumor marker used in routine disease diagnosis and follow-up.
Based on this information, we think that even if locally advanced gastric cancer is not detected radiologically, in the presence of high CEA and FAR values, patients should be evaluated more sensitively in terms of neoadjuvant therapy and staging. Although CEA and FAR values give an idea about prognosis and survival, they are not sufficient to distinguish early-stage cases. Endoscopic USG should not be forgotten in addition to traditional diagnostic methods in cases where no elevation in CEA and FAR values is observed.
Our study is the study with the largest number of patients in the available literature, examining the effects of CEA and FAR measurements on TNM stage, number of metastic lymph nodes and prognosis in resectable gastric cancers. We found that the combination of CEA and FAR had a high predictive ability of disease-free survival and overall survival of gastric cancer patients.
This study was limited due to its retrospective design. In addition, since the data are from a single-center, our findings need to be confirmed by other centers as well. We were unable to distinguish between possible outcomes resulting from the difference between the types of surgery and the postoperative chemotherapy regimens. Current guidelines recommend neoadjuvant chemotherapy for patients with locally advanced gastric cancer, and patients receiving neoadjuvant chemotherapy were not included in this study. This study only considers preoperative CEA, Albumin, and Fibrinogen, and it remains unclear whether postoperative levels have predictive value. Larger sample sizes and prospective randomized studies are needed for validation.
High CEA and FAR values measured preoperatively are proportional to the TNM stage, tumor size, and the number of metastatic lymph nodes, by easy and cheap blood tests regarding CEA and FAR values; we can predict the status of the patients with gastric cancer. CEA and FAR values should be evaluated together with the results of pre-operative radiological imaging, and the treatment plan should be made according to all results. Considering survival and prognosis in patients with very high preoperative CEA and FAR values, neoadjuvant chemotherapy should also be considered. We believe that pre-operative FAR and CEA values are independent predictors of survival. FAR and CEA are potential prognostic indicators for resectable gastric cancer due to their easy access and low cost.
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
[Crossref] [Google Scholar] [Pubmed]
Journal of Blood & Lymph received 443 citations as per Google Scholar report