Research Article - (2021) Volume 8, Issue 5
Received: 06-Sep-2021
Published:
01-Oct-2021
, DOI: 10.37421/bset.2021.8.119
Citation: Donthireddy Sushma. "Formulation and Evaluation
of Press Coated Tablets for Pulsatile Drug Delivery." J Biomed Syst Emerg
Technol 8 (2021): 119.
Copyright: © 2021 Donthireddy Sushma. This is an open-access article
distributed under the terms of the Creative Commons Attribution License, which
permits unrestricted use, distribution, and reproduction in any medium, provided
the original author and source are credited.
The aim of present investigation is to develop press coated tablet, a dry-coated device for pulsatile drug delivery of a pain relieving drug using different hydrophilic and hydrophobic polymers. It is intended to be used mainly in the therapy of pain symptoms which depend on circadian rhythms. The drug delivery system will be designed to deliver the drug at such a time when it could be most needful to patient of rheumatoid arthritis and fibromyalgia [1]. It will be aimed to have a lag time of four hours so that, the system is taken at the bed time and expected to release the drug after a period of 4 hours at mid night time when such pain are at peak and disturbs sleep. Such time-controlled pulsatile drug delivery can be achieved mainly with cores containing Tapentadol hydrochloride as active agent. Core formulations, characterized by different release rates and mechanisms, will be coated by compression with an outer shell of different polymeric barrier layers of hydrophobic and hydrophilic polymers. The coatings prevent drug release from the core until the polymeric shell is completely eroded or swollen. The release profile of press coated tablet is supposed to exhibit a lag time followed by burst release, in which outer shell ruptured into two halves. The dissolution profiles of uncoated cores and press-coated devices will be compared. Various evaluation tests will be carried out to ensure the best quality formulation. The factors influencing on lag time such as type of polymeric shell and outer coating weight will be also investigated. The surface morphology of the tablet was examined by a scanning electron microscopy [2]. Differential scanning calorimeter and Fourier transformed infrared spectroscopy study showed compatibility between drug and coating material.
Pregabalin • Matrix • Non-erodible • Erosion • HPMC • Plugs.
Press coating technique is a simple and unique technology used to provide tablets with a programmable lag phase, followed by a fast, or rate-controlled, drug release after administration. The technique offers many advantages, and no special coating solvent or coating equipment is required for manufacturing this type of tablet. Pulsatile drug delivery systems (PDDS) have attracted attraction because of their multiple benefits over conventional dosage forms. They deliver the drug at the right time, at the right site of action and in the right amount, which provides more benefit than conventional dosage and increased patient compliance. The dissolution profiles of uncoated cores and press-coated devices will be compared. Various evaluation tests will be carried out to ensure the best quality formulation. The surface morphology of the tablet was examined by a scanning electron microscopy [3]. Differential scanning calorimeter and Fourier transformed infrared spectroscopy study showed compatibility between drug and coating material. Circadian rhythms are self-sustaining, endogenous oscillations that occur with a periodicity of about 24 Hours. Interestingly, the term circadian is derived from the Latin circa which means about and dies which can be defined as a day. Normally, circadian rhythms are synchronized according to internal biologic clocks related to the sleep-wake cycle. Our circadian rhythm is based on sleepactivity cycle and is influenced by our genetic makeup and thereby affects our bodies function throughout day and night (24-hour period). Circadian rhythm regulates many body functions in humans like metabolism, physiology, behavior, sleep pattern, hormone production.
Preparation of core tablets by using direct compression
A direct compression method was used to prepare core tablet. All the ingredients like microcrystalline cellulose hydroxypropylemethyl cellulose, and directly compressible agent were sieved through no. 40 mesh screen and mixed uniformly using tumbler mixer [4]. Magnesium stearate and Talc was introduced into the blend and mixed properly then compressed into tablets using flat faced punches (Rotary tablet press, Karnavati Engineering) by keeping hardness 4 to 6 kg/cm2. The core tablets were evaluated for thickness, content uniformity, friability and disintegration.
Formulation of mixed blend for barrier layer
The various formulation compositions containing Ethylcellulose and L-HPC were weighed dry blended at about 10 min. and used as press-coating material to prepare press-coated pulsatile tablets respectively by direct compression method.
Preparation of press-coated tablets
The core tablets were press-coated with 400mg of mixed blend/granules and 200 mg of barrier layer material was weighed and transferred into a 13 mm die then the core tablet was placed manually at the center [5]. The remaining 200 mg of the barrier layer material was added into the die and compressed at a pressure of 5 tons for 3 min.
Pre-compression tests
Based on the results of pre-compression tests, formulation showed angle of repose 36.50 indicating a good flow property and Carr’s index is 20.92, indicating compressibility of the Blend is fairly passable (Table 1).
| Parameters | Mean readings |
|---|---|
| Bulk Density | 0.65 |
| Tapped Density | 0.84 |
| Carr’ index | 20.92 |
| Hausner ratio | 1.34 |
| Angle of repose | 36.5 |
In vitro drug release study of press-coated tablets
In-vitro dissolution studies of press coated tablets were performed at 37 ± 0.5°C using 0.5% w/v aqueous solution sodium lauryl sulfate in USP II paddle method at 50 rpm. 5 ml of filtered aliquot was manually withdrawn at pre-determined time intervals and replaced with 5 ml of fresh 0.5% sodium lauryl sulfate solution maintained at the same temperature. The samples were analyzed at 342 nm using a UV spectrophotometer. The lag time and percentage release was determined of the each formulation [6].
Drug Release Kinetics Study
Weight variation: The weight of the tablet being made was routinely determined to ensure that a tablet contains the proper amount of drug. The USP weight variation test is done by weighing 20 tablets individually, calculating the average weight and comparing the individual weights to the average (Table 2).
| S.NO | Average weight of Tablet (mg) |
Maximum % difference allowed |
|---|---|---|
| 1. | 130 or less | 10 |
| 2. | 130-324 | 7.5 |
| 3. | 324< | 5 |
Tablet hardness: The resistance of tablets to shipping or breakage under conditions of storage, transportation and handling before usage depends on its hardness. The hardness of each batch of tablet was checked by using Monsanto hardness tester. The hardness was measured in terms of kg/ cm2. 3 tablets were chosen randomly and tested for hardness. The average hardness of 3 determinations was recorded.
Friability: Friability generally refers to loss in weight of tablets in the containers due to removal of fines from the tablet surface. Friability generally reflects poor cohesion of tablet ingredients. 20 tablets were weighed and the initial weight of these tablets was recorded and placed in Roche friabilator and rotated at the speed of 25 rpm for 100 revolutions. Then tablets were removed from the friabilator, dusted off the fines and again weighed and the weight was recorded.
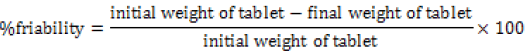
Evaluation of core tablets
From the above evaluation studies of pre-compression parameters, it was observed that the core formulation showed acceptable results. The angle of repose, hausner’s ratio values proved that the core tablet mixture was good in flowability as per the standard table of angle of repose, cars index and hausner’s ratio. The bulk density and tapped density were found to be nearer. There by it can be concluded that the pakagibility of compressed tablets is not affected much. There by the powder mixture can be used for their compressing to tablet for further development of press coated tablet (Table 3).
| Pre compression evaluation studies of core tablet | |
|---|---|
| Angle of repose | 26.565ᵒ |
| Hausner’s ratio | 1.20 |
| Carr’s index | 16.56° |
| Tapped density | 1.40 g/ml |
| Bulk density | 1.69 g/ml |
Post compression parameters
After compression of core tablet the tablets were evaluated for thickness,hardness, disintegration, friability, and drug content studies. Thickness of the core tablets were found to be which shows suitability to be compress coated keeping the core tablet at the center to obtain a suitable size of final press coated tablet. The hardness was found to be approximately 2-3 kg/cm2 on average showing optimum hardness for a core tablet to be compressed coated again. The disintegration time was found to be nearly 5.2 min average which may be because of high binding capacity of the polymers used in the core tablet formulation. The friability was found to be less than 1% for the core tablets evaluated. The drug content was found to be 94.9% on average for all the 3 core tablets which was acceptable to carry on further studies [7].
The post compression studies of press coated tablets included hardness test, thickness test, average weight test, swelling index test and lag time studies included of all press coated tablets was found to be acceptable from 6.2 to 7.8 kg/cm2. Thickness was found to be increasing with increase in the weight of the formulation. The acceptance limits as per Indian pharmacopeia. All the formulations showed uniformly of weights with least deviation within acceptable limits. The swelling index was found to vary according to polymers used to main contribution towards swelling index of all formulations was because of HPMC K100 polymer coat powder blend. It was found to range from 42-78%.
Dissolution studies results
The dissolution drug release profile is shown for the selected best chosen core tablet which was used for the preparation of all press coated tablet formulations [8]. The core tablet formulation showed 80% release in nearly 7.225 hrs. Which proved that the core tablet formulation was suitable for extended release of the drug? Similar to the marketed extend release formulation. The drug release followed zero order kinetics as R2 and K values are higher than other kinetics. The mechanism of drug release follows fikian diffusion.
The drug release profile of all PCT formulation shown that optimum results were optimizes from the formulation F3, F4, F6. Were the drug released for an extended period of time nearly 12 hrs. as can be seen in table no 6.7. As well as the lag time in this formulations were found to be optimum that is 4-4.5 hrs. as desired (Graph 1).
The F3 and F6 contain 50mgs HPC and 75 mgs of HPC respectively whereas F4 contain 75 mgs of ethyl cellulose the lag time obtain for formulation using eudragit were not justifying as well as eudragit were found to extend drug release be on 12 hrs (Graph 2).
The present investigation was based on formulation, development and evaluation of press coated tablets of Tapentadol hydrochloride in the study it was observed that the polymers used in the investigation were all erodible polymers. All polymers were able to provide lag time in the release of drug from the formulations. But among all polymers HPC and EC were found to be suitable in formulation of press coated tablet as they provide a predictable and repeatable same lag time in drug release for all formulations use in this. Among 12 formulations prepared EC, HPC and Eudragit in different proportions, the formulations F3, F4 and F6 were found to be best in terms of all evaluation parameters. The pre-compression evaluation parameters included Carr’s index, Hauser’s ratio, bulk density, tap density and angle of repose. The angle of repose, Hauser’s ratio values proved that the core tablet mixture was good in flow ability as per the standard table of angle of repose, cars index and Hauser’s ratio. The bulk density and tapped density were found to be nearer.
The core tablet formulation showed 80% release in nearly 7.225 hrs. Which proved that the core tablet formulation was suitable for extended release of the drug? Similar to the marketed extend release formulation. The drug release followed zero order kinetics as R2 and K values are higher than other kinetics. The post-compression evaluation parameters included thickness, weight variation, hardness, disintegration, friability, drug content studies. Thickness of the core tablets were found to be which shows suitability to be compress coated keeping the core tablet at the center to obtain a suitable size of final press coated tablet. The hardness was found to be approximately 2-3 kg/cm2 on average showing optimum hardness for a core tablet to be compressed coated again. The disintegration time was found to be nearly 5.2 min average which may be because of high binding capacity of the polymers used in the core tablet formulation. The friability was found to be less than 1% for the core tablets evaluated. The drug content was found to be 94.9%.
The post compression studies of press coated tablets included hardness test, thickness test, average weight test, swelling index test and lag time studies included of all press coated tablets was found to be acceptable from 6.2 to 7.8 kg/cm2. Thickness was found to be increasing with increase in the weight of the formulation. The swelling index was found to vary according to polymers used to main contribution towards swelling index of all formulations was because of HPMC K100 polymer coat powder blend. It was found to range from 42-78%. The drug release profile of all PCT formulations shown that optimum results were obtained from the formulation F3, F4, F6. Where in, the drug released for an extended period of time of nearly 12 hrs.As well as the lag time in this formulations were found to be optimum that is 4-4.5 hrs. as desired. The F3 and F6 contain 50 mgs HPC and 75mg of HPC respectively whereas F4 contain 75mgs of ethyl cellulose. The lag time obtained for formulations using Eudragit S-100 were not satisfying as well as Eudragit was found to extend drug release beyond 12 hrs. Based on the present study, it can be concluded that, the HPC and EC were the best polymers at the medium concentrations, in the development of PCT for the drug.