Research Article - (2024) Volume 9, Issue 1
Received: 10-Jan-2024, Manuscript No. Arwm-24-124871;
Editor assigned: 12-Jan-2024, Pre QC No. P-124871;
Reviewed: 24-Jan-2024, QC No. Q-124871;
Revised: 29-Jan-2024, Manuscript No. R-124871;
Published:
05-Feb-2024
, DOI: 10.37421/2475-7675.2024.9.321
Citation: Khemakhem, Marwa, Clara Le Rouzic, Thierry Falher and Valentin Thoury, et al. “Mechanical Recyclability Potential of Biobased and Biodegradable Poly (Hydroxybutyrate)/Poly (Butylene Succinate-Co-adipate) Blends.” Adv Recycling Waste Manag 9 (2024): 321.
Copyright: © 2024 Khemakhem M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The effect of repeated injection processing cycles on the structure and properties of poly (hydroxybutyrate)/ poly (butylene succinate-co-adipate) blends, initially extruded by using a twin screw extruder, was investigated. The recycling was simulated by performing five successive injections/ grinding runs. Neat poly (hydroxybutyrate) was also reprocessed under the same conditions as a reference. The results showed that the recyclability of PHB is restricted due to its sensitivity to thermomechanical degradation. Nevertheless, the PBSA addition had a stabilizing effect on PHB and an improved compatibility between the two components was observed during the reprocessing cycles due to a morphology evolution from cocontinuous to droplets in matrix type. This morphological transition was the main factor to the significant increase of the blend ductility right after the first injection cycle.
The thermal, the viscoelastic and the mechanical properties of this blend were maintained during the recycling process thus testifying that this formulation can be recycled several times by injection molding without losing its performances before being composted.
PHB • PBSA • Recycling • Injection molding • Compatibility
Poly (Hydroxybutyrate) (PHB) is an aliphatic polyester which belongs to the large family of Polyhydroxyalkanoates (PHAs) [1,2]. It is a semi-crystalline thermoplastic with biodegradable, compostable and biocompatible properties, produced by several bacteria as intracellular carbon reserve [3,4]. However, some major disadvantages, including low thermal stability and inherent brittleness as well as high costs, may limit its widespread applications [5]. Meanwhile, according to European bioplastics, the global annual production of bioplastics is expected to rise from 2.23 Mt in 2022 to approximately 6.3 Mt in 2027, which is likey to reduce their costs [6].
Although PHB is considered biodegradable, recycling has emerged as the preferred alternative, according to the European Directive on waste management [7], because the life cycle of every plastic material is considered to be sustainable provided that its disposal options include recycling. After the prevention and reuse option, mechanical recycling is the best alternative for biobased plastics, followed by chemical recycling for materials that cannot be mechanically recycled [8].
PHB is known to be very sensitive to thermal degradation during melt processing due to the narrow temperature window between melting temperature and degradation one [9].
The mechanical recycling of PHB is usually associated with a reduction in quality, such as loss of tensile strength and molecular weight [10,11].
The recyclability of PHB was evaluated by Rivas LF, et al. [12] through multiple processing cycles via extrusion, injection molding and compression molding. After each cycle, it was shown that the thermal and mechanical properties were related to the resulting polymer structure. The obtained results indicated a significant reduction in mechanical properties already at the second extrusion cycle, with a reduction above 50% in the third cycle. In therm of crystallinity degree, an increase was observed due to chemi-crystallization process during degradation by chain scission [12].
Shah B, et al. [13] focused on the ability of PHB copolymer to be reprocessed by using different virgin to regrind ratios. A reduction by 79% in viscosity and 10% in ultimate tensile strength was observed after 10 regrind generations. A drop of 5% in the viscosity and ultimate tensile strength was also observed with a 50/50 virgin to regrind ratio.
Extrusion and injection molding were perfomed by Zaverl M, et al. [14] to study the recyclability of PHBV. After four extrusion cycles, the molecular weight was decreased from 337 to 281 KDa due to thermal degradation and the crystallinity rate was reduced from 54% to 42%. The tensile and flexural strengths were decreased by 7.1% and 8.3%, respectively, while the same impact toughness value was kept.
It has been reported that the thermal degradation of PHB, upon thermal processing, predominanatly consists of random β-elimination scission, inducing the formation of crotonic acids and oligomers with crotonate endgroups [15]. β-elimination scission produce a large number of carboxylic compounds that randomly attack ester groups on polymer chains to induce transesterification reactions, resulting in the reproduction of carboxyl groups which auto-catalyze the random degradation [16].
The aforementioned studies highlighted the structural degradation of PHB during the recycling process under the effect of thermomechanical treatment. Added to its inherent brittleness, the thermomechanical sensitivity of PHB might limit its use for large-scale applications.
To overcome these drawbacks, many approaches have been established, including copolymerization [17] and blending with other polymers. Polymer blending is an economic and efficient strategy to develop new polymeric materials with desirable properties.
Many studies have focused on blending PHB with other polymers like Polylactide (PLA) [18], Poly (ε-CaproLactone) (PCL) [19-21], Poly (Butylene Adipate Terephtalate) (PBAT) [21,22], Poly (Butylene Succinate) (PBS) [23- 25] and Poly (Butylene Succinate-Co-Adipate) (PBSA) [23,26].
PBS is a very interesting aliphatic polyester, partially or fully biobased [27], synthesized by the polycondensation of 1,4-butanediol and succinic acid. PBS and its copolymer PBSA exhibit balanced mechanical properties similar to those of polyolefins, high chemical resistance and good melt processability [28]. Therefore, blending PHB with PBSA offers the possibility of improving the thermomechanical properties and subsequently the recyclability of PHB.
The addition of PBSA to PHB was found to decrease the intrinsic brittleness of PHB thus improving the material ductility [23], the physical adhesion between the two components was explained by the co-continuous morphology and the sequential crystallization of the two polymers.
A poor compatibility between PHBV and PBS was proven and led to low tensile strength [29]. However, the moduli of blends were significantly enhanced compared to the neat PBS, whereas the elongation at break values of the blends were higher than that of the neat PHBV.
Recently, Peshne H and Satapathy BK [30] comparatively evaluated PHB/ PBS and PHBV/PBS blends for their thermomechanical, morphological and rheological properties. The crystallization of PHB and PHBV was restricted in the presence of PBS whereas the thermal stability of the blends was enhanced. When the PBS content increased from 10 to 50 wt%, a change in the morphology from domain dispersed to co-continuous type happened.
Nevertheles, up to now few efforts have been dedicated to the study of mechanical recyclability of these promising blends. Chikh A, et al. [31] investigated the recyclability of PHBV/PBS blend and PHBV/PBS/ sepiolite nanocomposites with and without compatibilizer by assessing the effect of successive extrusion cycles on the thermal, morphological, rheological and mechanical properties. The obtained results revealed that the the thermomechanical degradation of PHBV was strongly reduced in the presence of PBS, but more pronounced in the presence of both sepiolite and compatibilizer. On the other hand, the elongation at break was still low (about 5%), even upon the addition of PBS, and further decreased during the reprocessing cycles of the PHBV/PBS blends.
The present work aims to assess the recyclability potential of PHB/PBSA (50/50) (wt%) blend by studying the effect of repeated injection molding cycles on the structure, physical and rheological properties of the recycled materials. Neat PHB was also reprocessed as a reference material.
Materials
PHB (commercial grade ENMAT Y3000P) was acquired from Tianan Biologic Material Co. Ltd. (China) in pellets form. Bio-based PBSA (commercial grade BioPBS FD92PM/FD92PB) is produced from the polymerization of biobased succinic acid, 1,4-butanediol and adipic acid and supplied by PTT MCC Biochem Co. (Thailand).
Melt processing and reprocessing
PHB and PBSA pellets were dried at 60 ℃ for 24 h prior to melt processing (extrusion or injection).
The neat PHB was directly subjected to successive recycling cycles by injection molding in an Engel 150T injection machine press. The temperature was set to 160, 165, 165, 170, 175 ℃ along the five sections of the press, from hopper end to nozzle. The temperature of the mold was kept at 20 ℃, the injection pressure was 650 bars and the hold time in the mold was 15 s. The injection flow rate was set to 60cm3/s corresponding to a shear rate of 6700s-1.
A number of the injected dumbbell-shaped specimens was used for the characterizations, while the other ones were ground to obtain new rigid flakes for the further additional injection molding steps, enumerated from 1 to 5.
The PHB/PBSA (50/50) (wt%) blend was firstly compounded by using a twin-screw extruder Coperion ZSK32 with a L/D ratio of 40 and a screw diameter of 32 mm. The rotation speed was fixed to 105 rpm, corresponding to a shear rate of 350 s-1. The temperature profile along the extruder, from the feed zone to the die, was set to 80, 145, 155, 165, 165, 165, 165, 180, 180, 180 ℃.
Then, the obtained strands were air cooled and pelletized. A set of five successive injection cycles was performed under the same processing conditions described above.
Characterization
Differential Scanning Calorimetry (DSC): The thermal properties of neat PHB, neat PBSA as well as PHB/PBSA blend after each processing cycle were investigated with the help of a differential scanning calorimeter Q100_ DSC (TA) equipped with a Liquid Nitrogen Cooling System (LNCS). The DSC cell was constantly purged with nitrogen at a flow rate of 50 mL/min. A set of heating/cooling ramps was carried out following a three-step process; the samples were firstly heated to 200 ℃ at 20 ℃/min and kept in the molten state for 1 min to erase the thermal history of the material. They were then cooled down to -70 ℃ at 10 ℃/min to evaluate the ability of both polymers to crystallize upon cooling. After cooling treatment, the samples were heated back to 200 ℃ at 10 ℃/min. The percent crystallinity was calculated upon the second heating by using the following equation (Eq. 1):
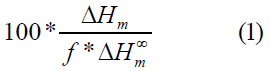
Where “ΔHm” is the measured heat of fusion, “f” is the weight fraction of PHB or PBSA in the blend and “ΔH∞m” is the enthalpy of fusion for a crystal having infinite crystal thickness (113.4 J g-1 for PBSA [32] and 146 J g-1 for PHB [31]).
Thermo-Gravimetric Analysis (TGA): Thermal Gravimetric Analysis (TGA) studies were performed using a Mettler Toledo TGA analyzer under nitrogen at a flow rate of 50 mL/min. Samples (10-15 mg) were heated from 25 ℃ up to 800 ℃ using a heating rate of 10 ℃/min.
Scanning Electron Microscopy (SEM): The microstructure of samples was examined by Scanning Electron Microscopy (SEM) using a Zeiss FEG microscope (SUPRA 55VP). Accelerating voltage was maintained constant at 6 kV. The samples were surfaced by a cryo-ultramicrotome (LEICA UC7), where both knife and specimen were cooled by liquid nitrogen. Then, the flat surfaces were metallized prior to observations.
Molecular weight measurement: Size Exclusion Chromatography (SEC) was used to follow the evolution of the PHB molecular weight during the reprocessing cycles.
The chromatograph is equipped with a pre column PLgel 5 μm Guard 50 × 7.5 mm, a set of three columns PLgel 3 μm MIXED-C 300 × 7.5 mm and a refractive index detector.
PHB solutions were prepared as 3 mg/mL in Chloroform, with a flow rate of 1 mL min-1.
The calculations were based on calibration curves obtained from polystyrene standards with different molar masses.
The weight-average molecular weight 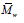 and number-average molecular weight
and number-average molecular weight 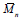 are obtained from the SEC analysis. The polydispersity index was calculated as
are obtained from the SEC analysis. The polydispersity index was calculated as 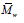 /
/ 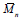 .
.
Melt rheological study: Low Amplitude Shear measurements (LAOS) were investigated with an ARES G2 rotational rheometer at a temperature of 180 ℃, using a parallel plates geometry with a plate diameter of 25 mm and a gap of 1 mm. Prior to experiments, dynamic strain sweep tests with a maximum angular frequency amplitude of 100 rad/s were performed.
Hence, the strain value is set at 2% to validate the linear viscoelastic region and the dynamic oscillatory shear measurements were carried out at the frequency range 0.1-625 rad/s.
Mechanical properties: Mechanical tests were conducted using MTS criterion machine equipped with an extensiometer. Tensile experiments were performed at room temperature with a cross-head speed of 1 mm/min in the deformation range of 0-3% and 50 mm/min when the deformation exceeded 3%.
The average of five tested specimens for each material was reported.
Molecular weight measurements
The changes in the weight average molecular weight (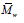 ), the number
average molecular weight
), the number
average molecular weight 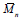 and the polydispersity index (
and the polydispersity index ( 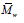 /
/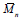 ) occuring
during the melt processing and reprocessing by injection molding are
presented in Table 1.
) occuring
during the melt processing and reprocessing by injection molding are
presented in Table 1.
| Sample | 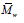 (Da) (Da) |
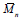 (Da) (Da) |
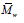 / /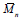 |
|---|---|---|---|
| Neat PHB | 3,33,099 | 1,24,343 | 2.68 |
| PHB1 | 2,10,947 | 98,512 | 2.14 |
| PHB2 | 1,56,489 | 74,962 | 2.08 |
| PHB3 | 1,53,550 | 74,018 | 2.07 |
| PHB4 | 1,47,824 | 69,104 | 2.14 |
| PHB5 | 1,40,242 | 71,405 | 1.96 |
It can be seen that ( 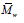 ) as well as (
) as well as (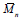 ) of PHB sharply decreased by 37%
after the first injection cycle (sample PHB1), which indicates the occurrence of
polymer degradation. Then, after the second injection cycle (sample PHB2),
) of PHB sharply decreased by 37%
after the first injection cycle (sample PHB1), which indicates the occurrence of
polymer degradation. Then, after the second injection cycle (sample PHB2), 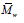 was reduced by 26%, thus confirming the predominance of chain scission
mechanism as a result of degradation.
was reduced by 26%, thus confirming the predominance of chain scission
mechanism as a result of degradation.
For PHB polymer, the significant reduction in molecular weight during the two first injection cycles can be attributed to the unzipping reaction in PHB through cis-elimination mechanism just above its melting temperature [33].
From the third injection cycle up (sample PHB3) to the last injection cycle
(sample PHB5), slight variations in 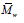 and
and 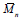 could be noticed. Therefore,
the main degradation process took place during the two first injection cycles
could be noticed. Therefore,
the main degradation process took place during the two first injection cycles
Differential Scanning Calorimetry (DSC)
DSC analysis was performed in order to assess the thermal properties of PHB after each injection cycle as well as the PHB/PBSA (50/50) blend.
The thermal characteristics of PHB and PBSA polymers after each reprocessing cycle, in terms of glass transition temperature (Tg), melting temperature (Tm), heat of melting (ΔHm), crystallization temperature (Tc) and crystallization enthalpy (ΔHc) are summarized in Table 2 while Figure 1 and Figure 2 display the obtained thermograms of the second heating cycle and the cooling cycle for PHB and PHB/PBSA blend, respectively (Figures 1 and 2 and Table 2).
| (PHB/PBSA) Samples | Designation | Tg onset(°C) | Tm(°C) | ΔHm(J.g-1) | Tc(°C) | ΔHc(J.g-1) | χc(%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PHB | PBSA | PHB | PBSA | PHB | PBSA | PHB | PHB | PHB | PBSA | PHB | PBSA | ||
| X(100/0) | Unprocessed | -0.9 | - | 175.6 | - | 96.6 | - | 123.6 | - | 91.1 | - | 66 | - |
| Injection1 | -1.3 | - | 175.8 | - | 96.3 | - | 124.6 | - | 89.4 | - | 66 | - | |
| Injection2 | -1.4 | - | 174.8 | - | 96.7 | - | 124.5 | - | 86.95 | - | 66 | - | |
| Injection3 | -1.1 | - | 175.2 | - | 92.6 | - | 124 | - | 86.2 | - | 63.4 | - | |
| Injection4 | -0.2 | - | 174.2 | - | 92.5 | - | 124.4 | - | 84.8 | - | 63 | - | |
| Injection5 | -0.4 | - | 173.9 | - | 92.8 | - | 123.4 | - | 85.9 | - | 63.5 | - | |
| (50/50) | Compound | - | -47 | 174.2 | 86.9 | 44.4 | 15.1 | 113.3 | 50.3 | 38.3 | 16.4 | 60.8 | 26.5 |
| Injection1 | - | -48.6 | 173.1 | 85.8 | 44.8 | 16.3 | 113.5 | 49 | 39.2 | 17.9 | 61.3 | 28.7 | |
| Injection2 | - | -48.8 | 171.4 | 86.1 | 41.4 | 15 | 110.7 | 48.7 | 37.5 | 18.1 | 56.7 | 26.4 | |
| Injection3 | - | -47.7 | 172.7 | 85.9 | 47.9 | 17.5 | 111.9 | 51.1 | 43 | 20 | 65.6 | 30.9 | |
| Injection4 | - | -44.8 | 173 | 86 | 45.1 | 18.2 | 110 | 48.1 | 40.9 | 19.9 | 61.8 | 32.1 | |
| Injection5 | - | -45.5 | 172.9 | 86.1 | 48.2 | 18.6 | 109.9 | 48.4 | 41.7 | 20.9 | 66 | 32.8 | |
The thermal properties of PHB remain practically unchanged during the injection successive cycles so that there is no indication of degradation phenomenon.
However, when blended with PBSA, the PHB undergoes some changes in its thermal properties, namely after the second injection cycle, where a decrease in the melting temperature was noticed as compared to the sample deriving from the compounding step, attesting for a less perfect crystals. In parallel, when comparing the crystallization temperature of PHB in the neat material to the crystallization temperature of PHB in the blend, a sharp decrease could be seen (from 124 ℃ to 113 ℃) and this temperature further decreased during the reprocessing cycles of the blend. This result indicates that the presence of PBSA partially hindered the crystallization of PHB phase. These findings suggest an assisted heterogeneous crystallization between the two polymers, becoming more important by succeeding the reprocessing cycles, which means that the compatibility between PHB and PBSA is enhanced through the recycling process.
Additionnaly, the crystallinity level of PHB in (PHB/PBSA) blend was lower than that of the neat material, thus demonstrating that the presence of PBSA restricted the crystal growth of PHB. This result is likely due to the existence of molten domains of PBSA near the crystallization growth front, which can disturb the crystal growth of PHB [34,35].
These thermal modifications can be associated to the interactions with PBSA, which decreased the macromolecular chain mobility and consequently reduced the level of crystallinity for PHB.
During the injection reprocessing cycles of the (50/50) blend, a sharp decrease of the crystallinity degree of PHB phase was observed after the second injection cycle. However, from the third cycle, the crystallinity rate becomes close to that of neat PHB, probably due to the recrystallization of PHB molecular segments deriving from the thermo-mechanical degradation during the injection process [36].
Thermo-Gravimetric Analysis (TGA)
The thermal stability of the samples was studied by TGA. The weight loss of each polymer, the onset temperature of the decomposition, the temperature corresponding to the maximum weight loss rate (Tmax) obtained from the Derivative Weight Loss curve (DTG) and the char yield at 800 ℃ are summarized in Table 3.
| (PHB/PBSA) Samples | Cycle Designation | Weight Loss (%) | T degradation onset (℃) | Tmax (℃) | Char Yield (%) | |||
|---|---|---|---|---|---|---|---|---|
| PHB | PBSA | PHB | PBSA | PHB | PBSA | |||
| (100/0) | Injection 1 | 98.8 | - | 268 | - | 285 | - | 1.2 |
| Injection 2 | 99.2 | - | 264 | - | 284.7 | 0.84 | ||
| Injection 3 | 98.9 | - | 266 | - | 284.7 | 1.1 | ||
| Injection 4 | 99.2 | - | 270 | - | 285 | 0.8 | ||
| Injection 5 | 98.2 | - | 271 | - | 287.3 | 1.8 | ||
| (50/50) | Compound | 51.4 | 47.2 | 268 | 341.6 | 283.2 | 385 | 0.85 |
| Injection 1 | 49.3 | 49.9 | 272 | 351.8 | 285.4 | 395 | 0.6 | |
| Injection 2 | 48.6 | 50 | 276 | 354 | 290 | 394 | 1.1 | |
| Injection 3 | 49.8 | 49.9 | 271 | 359 | 283.4 | 389 | 0.5 | |
| Injection 4 | 49.7 | 50.2 | 268 | 351 | 280.6 | 381 | 0.6 | |
| Injection 5 | 49.6 | 50 | 267 | 342.9 | 280.3 | 376 | 0.34 | |
The TGA results show that after the decomposition of PHB a residue of about 1% is left thus suggesting the presence of inorganic particles such as nucleating agents [37,18].
During the successive injection cycles of the PHB polymer, it can be noticed that its characteristic degradation temperatures (Tonset and Tmax) remain unaffected.
On the other hand, for the (PHB/PBSA) (50/50) blend, the onset degradation temperature and the maximum degradation temperature of the PHB component increased after the first and the second injection reprocessing cycles, in comparison to the PHB degradation temperatures after the compounding step. This trend reveals that the PBSA component has improved the thermal stability of the PHB due to interactions between PHB and PBSA macromolecular chains by going from one cycle to another during the recycling process. This result is in accordance with the literature [38] where blending PHB with PBS was proved to improve the thermal stability of PHB phase.
Nevertheless, from the third injection cycle, the maximum degradation temperature of PHB in the blend significantly decreased, likely due to chain scission, which is consistent with the DSC results.
Morphology of the blends
As the properties of polymer blends are strongly dependent on their structure, it is important to understand the basic mechanisms of the morphology development, which constitutes the result of the dynamic equilibrium between break-up and coalescence of the droplets.
Taylor analysis [39,40] considers that the final morphology of a polymer blend depends on several parameters involving the composition of the blend, the interfacial tension, the viscosity ratio and the processing parameters.
These parameters can be expressed by the ratio between the capillary number Ca and the critical capillary number Cacr.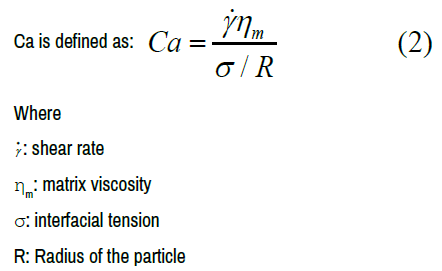
Cacr is derived from the flow kinematics and the viscosity ratio and defined as follows:
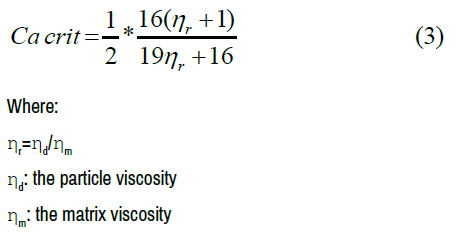
According to Grace HP [41], the break up can occur when the caplillary number Ca exceeds a critical capillary number Cacr (imposed by the ratio between doplet viscosity and matrix viscosity). The lower value of the capillary number is obtained for a viscosity ratio close to 1. For a viscosity ratio higher than 1 or lower than 1, a higher capillary number is required for the rupture of the drops.
In this context, the morphology development of PHB/PBSA (50/50) compounded blend as well as the blends deriving from the successive injection molding steps was examined using scanning electron microscopy. The observations were perfomed on the surface of a compression molded plate for the compounded blend and on the surface of injected tensile specimens from the different injection cycles.
Figure 3 illustrates the topology of the blends at X500 and X1500 magnifications (Figure 3).
It can be seen on the obtained micrographs that the compounded blend exhibits a co-continuous morphology, with two interpenetrated phases. Moreover, in some regions the voids between the two separate phases were clearly visible. These observations indicate the weak interfacial adhesion between the two phases, thus low mechanical properties are expected for this sample.
Conversely, the morphology of the blend deriving from the first injection cycle appears different: A typical matrix-droplets morphology was observed and the separation of the two components is less evident.
The change in the phase morphology of the PHB/PBSA (50/50) blend is caused by the evolution of the (critical) capillary number of the blend. In fact, the shear produced during the injection process (shear rate=6700s-1) enables the capillary number Ca to exceed the critical capillary number Cacr. The PBSA phase domains were then deformed, extended and broken into small droplets. Therefore, the PBSA tended to be the dispersed phase with a fine dispersion and a homogeneous distribution. A substantial improvement of the mechanical properties, namely the toughness, can be predicted for this morphology type.
On the other hand, the shear produced during the extrusion process (shear rate=350 s-1) shifted the dynamic equilibrium towards the coalescence phenomenon which is produced through collisions between dispersed polymer droplets.
Such an effect was observed by other researchers [42,43] who considered that the shear rate is a key parameter control.
When comparing the micrographs of the blends deriving from the 1st, the 3rd and the 5th injection cycles, no significant differences could be seen, revealing that the morphology of the injected samples was not affected by the reprocessing cycles.
Melt rheological study
Rheological characterizations were used to investigate the effect of repeated injection processing cycles on the viscoelastic properties of neat PHB and its blend with PBSA under real melt conditions. The complex viscosity and the storage modulus are presented as a function of angular frequency for neat PHB after different injection processing cycles and (PHB/PBSA) blend deriving from the compounding step as well as the successive injection cycles (Figures 4 and 5).
The viscosity-frequency curves of PHB melt display a maximum instead of a horizontal plateau in the low frequency range (Figure 4a). This unusual behavior can be related to a thermal degradation process at long residence times in the rheometer because the rheological measurements started from high down to low frequencies
It can be seen in Figure 4 that the complex viscosity of PHB was reduced when going from the first to the third injection cycle. This viscosity decrease can be correlated to a decrease in the polymer molecular weight. Then, the complex viscosity remained roughly unaffected when going from the third to the fifth injection cycle.
On the other hand, the (PHB/PBSA) blend has a non-Newtonian behaviour from low to high frequency, with viscosity increasing as frequency decreases (Figure 5a). By adding the PBSA component to PHB, the shear thinning behavior becomes more significant, representing a typical characteristic of a pseudoplastic fluid behavior.
The recycling process induces a significant raise of the complex viscosity of the blend. In fact, its value at 0.1 rad/s increased from 164 Pa.s for the compounded blend to 621 Pa.s for the blend resulting from the fifth injection cycle. This viscosity increase could be due to the creation of more molecular entanglements between PHB and PBSA chains by going from one cycle to another during the recycling process. The linkages between the two phases can occur via a combination of PHB and PBSA free radicals resulting from the - scission mechanism of the polyesters during the injection process [24].
The variation in storage modulus as a function of frequency is shown in Figure 5b. The elastic modulus of the (PHB/PBSA) (50/50) blend slightly increased after the first injection cycle with respect to the compounding step. Then, its value markedly increased after the third injection cycle and remains practically unchanged after the fifth injection cycle. In fact, at very low frequencies, the compounded blend exhibited a modulus of about 6 Pa while the blend deriving from the fifth injection cycle presented a value of around 33 Pa. This was a consequence of the physical network created between the two phases during the injection recycling process, thereby inducing the formation of an interphase which would consolidate the blend structure. The elastic modulus of the material in the molten state was thus greatly enhanced. These results are consistent with the morphological observations revealing the breakage of PBSA phase domains into smaller droplets during the injection reprocessing cycles, generating the increase of PHB/PBSA interface and therefore the overall increase of the blend elasticity.
In view of the obtained results, it can be concluded that the PBSA has a stabilizing effect on PHB. After blending with PBSA, the thermal stability of PHB was strongly improved. Moreover, during the reprocessing cycles, a better compatibility between the two components was generated due to more interactions between the macromolecular chains of both polymers.
Mechanical properties
The changes in the mechanical properties after the injection reprocessing cycles of PHB and PHB/PBSA blend were investigated using tensile tests. Tensile modulus, tensile strength at break and elongation at break are reported vs. reprocessing cycles in Figure 6.
It can be observed that the Young’s modulus of the PHB deriving from the first injection cycle is about 4.4 GPa, which is twice higher than the Young’s modulus obtained elsewhere for an extruded PHB [14]. The tensile strength value was also significantly higher than that obtained for an extruded PHB (39 MPa in injection and 25 MPa in extrusion).
These observations are probably assigned to the injection process parameters. In fact, the final properties of the molded product strongly depend on the processing conditions used during injection [44]. The mold temperature was reported as the parameter that mostly impacts the Young’s modulus, followed by the barrel temperature.
Additionally, the high shear forces during the injection process are likely to induce a significant molecular orientation in the flow direction and therefore impart strength to the material in this direction.
The elastic modulus as well as the tensile strength were maintained through the five injection cycles, it can be concluded that the PHB could withstand similar stress values and the recycled material could be reused as virgin material in the production line. However, the elongation at break of the PHB remain very low during the repeated injection cycles, even though other studies, devoted to the poly (Hydroxybutyrate-co-3-Hydroxyhexanoate), achieved an elongation at a break value of 330% at optimized processing conditions with high mold temperatures compared to 156% at non-optimal processing conditions with low mold temperatures [45].
For the (PHB/PBSA) (50/50) blend, the elongation at break increases from 3.2% for the compound processed by twin-screw extrusion to 20% for the blend after the first injection cycle and further increases to 26% after the fourth injection cycle. The reduction of brittleness is also highlighted by the decrease of the tensile modulus from 4.5 GPa for the injected neat PHB to 1.2 GPa for the (50/50) blend after the first injection cycle.
These findings can be related to the developement of finer PBSA domains due to high shear forces during the injection process. These forces were able to break the coalescence between the PBSA particles and therefore to promote the compatibility between PHB and PBSA components. As a result, the PBSA phase, due to its greater ability to absorb the energy required to break the sample, contributes to the improvement of the elongation at break of the material.
Unlike the study of Chikh A, et al. [31] a high elongation at break was reached here during the successive injection molding steps of the PHB/PBSA (50/50) blend thanks to the used injection molding process, which is able to provide higher shear rates than the extrusion process commonly used to evaluate the mechanical recyclability of bioplastics.
The toughness of the PHBV/PBSA blend in the above mentioned work was not improved, due to a complete phase separation and a poor interfacial adhesion between the two polymers even by performing repeated extrusion cycles.
Throughout this work, the recyclability of PHB biopolymer was shown to be limited due to a significant degradation of polymer chains after multiple reprocessing. The thermal degradation of PHB was demonstrated and quantified by using thermal and rheological tools coupled to molecular weight measurements.
However, the thermo-mechanical degradation of PHB was significantly reduced upon blending with PBSA. A size refinement of the PBSA phase domains within the PHB continuous phase during the injection recycling cycles was demontrated by morphological observations. This structure developement ensured a better compatibility between the two polymers which was responsible for the enhancement of the mechanical properties of the blend. An optimum balance of toughness and stiffness was thus obtained.
Therefore, it can be concluded that the PHB recyclability can be improved by blending with PBSA. The reprocessability was confirmed by the thermal measurements, the morphological study, the viscoelastic properties assessment and the mechanical tests.
All the measurements have shown that the materials properties were maintained during the five successive injection cycles. Therefore, the developed formulations can be reused at least for five processing cycles in injection molding as virgin materials.
These blends, being biobased and biodegradable, their end of life can be the biodegradation in domestic conditions, but the obtained results showed that the mechanical recycling option has to be also considered.
This work was supported by nenu2PHAr project and funded by the European Union’s Horizon 2020 Research and Innovation Program under grant agreement No. 887474.
The authors declare no conflicts of interest.
Advances in Recycling & Waste Management received 438 citations as per Google Scholar report