Research Article - (2024) Volume 8, Issue 4
Received: 01-Sep-2024, Manuscript No. jeh-24-147074;
Editor assigned: 03-Sep-2024, Pre QC No. P-147074;
Reviewed: 10-Sep-2024, QC No. Q-147074;
Revised: 17-Sep-2024, Manuscript No. R-147074;
Published:
24-Sep-2024
, DOI: 10.37421/2684-4923.2024.8.228
Citation: Alzahrani, Ahmed, Mohamed Hassan and Hussain
Alsalamah. “New Quality Index and Classification Criteria of Charcoal Based on
Experimental Results of Different International Standards.” J Environ Hazard 8
(2024): 228.
Copyright: © 2024 Alzahrani A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The purpose of this study is to promote consistency in national and international charcoal testing protocols. The methods employed for assessing volatile matter and ash content in charcoal are rooted in empirical approaches, exhibiting significant variations across different countries. Within this study, the volatile matter content was gauged under two distinct treatment conditions, namely ISO 562 and ASTM D1762, while ash content was ascertained under two distinct treatment conditions, i.e., ASTM D3174 and ASTM D1762, across varying heating rates. A comparison of various characteristics, including fixed carbon, volatile substance, gross calorific values and ash levels, was done on charcoal samples made from Deer brand charcoal, Coconut sawdust, Palm tree debris and unknown natural products. Variations in volatile matter and ash content were produced by the choice of the technique used. Each criterion demonstrates distinctive qualities that could have an impact on the results. The results indicate a strong correlation between the high volatile content of the charcoals and their low calorific values. Charcoal made from natural products, specifically Indonesian products and Deer brand charcoal have been found to have high calorific values of 31557.2 and 31403.9 KJ/Kg, low volatile matter at 6.47 and 12% and minimal ash content at 2.4 and 2.9%, according to research conducted using the ASTM D1762 technique. Based on the experimental results and different international classification of charcoal, we derived classification criteria and quality index for the quality check of charcoal samples.
Ash content • Calorimeter • Charcoal • Calorific Values • Quality index
Wood waste, vegetables, trees and agricultural leftovers are just a few examples of the wide variety of substrates for biomass that exist on Earth. To make solid biofuels, these feedstocks can either go through thermochemical and biochemical processes or be burned directly in a heating system. A variety of energy properties must be evaluated to gauge the potential of solid biofuels. Three key energy variables categorized dry biomass using proximate analysis include constant carbon and volatile substance, whole ash and total moisture [1]. However, it is cost effective, simple and frequently adopted to make use of muffle furnace technology for this purpose [2].
The amount of all the ash in the sample ranges from 0.1% to 10%, with greater amounts suggesting contaminant taints. Natural lignocellulosic residue typically contains 65 percent to 85 percent volatile matter. Biomass can contain between 15% and 25% fixed carbon, which is the major component of raw materials [3]. The biofuel releases water, tar, light hydrocarbons, carbon dioxide, carbon monoxide and hydrogen when heated. The combustion starts to ignite when flammable gases are present; nevertheless, the inability to reach high temperatures is due to the substantial amount of concentration of the gases present [3].
To measure the volatile matter and the amount of ash, a variety of test standards are available in the literature; the optimal standard to use depends on the kind of solid fuel being analyzed. The ASTM D1762 (1984- reapproved -2021) standard is more appropriate for determining the volatile matter and ash concentration in biofuels made from charcoal. Both the ASTM E872 (1982) standard for wood particles and the ISO18123 (2014) standard for solid biofuels use comparable test methods [4]. Previous studies on biomass characterization have already used ASTM D3175-2011, the specific standard designed for petroleum coke and mineral coal [5].
The Saudi standard No. SASO 2880:2017 & Gulf standard No. (GSO 2583:2021) [6] were issued to cover the requirements and test methods for charcoal derived from wood in the form of lump or briquette. The two standards depend on the testing methods used to judge the quality of coke and coal (ISO 562/2012 Hard coal and coke - Determination of volatile matter & ASTM D 3302/2019-for total moisture in coal) to be applied when judge the quality of charcoal. More over the test method used to measure, the ash content not fit the purpose of use as long as it deals with ash fusion (ISO 540 /2012-hard coal and coke-determination of ash fusibility).
Key characteristics of charcoal such as fixed carbon, the amount of ash, volatile matter and moisture are all determined by the aforementioned standards. When these characteristics are properly balanced, charcoal has the best quality possible. However, if these values are out of balance, the charcoal may burn either excessively slowly or too quickly. On top of that, burning charcoal creates more ash and volatile matter if the fixed carbon level is low. The high quantity of ash content in the charcoal indicates a significant presence of lignite, minerals, sand and other similar materials. This not only results in discomforting smoke but also makes it more challenging to start charcoal fires and alters the taste of food. Pure coke has a value of just under 33,000 KJ per kg, pure, dry wood has a net calorific value of 19,000 KJ per kg; for charcoal to ignite, a certain quantity of "volatile matter " must be present (EN 1860-2, 2005). By giving pressure to pulverized charcoal from wood, or other biomasses, or heat-treated mineral coal with the help of a binding substance such as corn flour is used to prepare briquettes. Peat-to-anthracite coal is an example of mineral coal, which is a by-product of plant fossilization. The type and grade of the coal used and the materials used in the compression process have an impact on the quality of the briquettes. Notably, it is observed that the ash content can change within the range of 4% to 40% [7]. Variations in procedural minutiae frequently have more impact than differences in core design. The specifics take on a great deal of significance given the experimental character of coal analysis methods. Thus, it is crucial to carefully examine processes with various characteristics to gauge the extent of their influence on outcomes and determine their applicability to a variety of coal types. One standard may differ from another in terms of granulometry, overall test time and how samples are arranged inside the equipment. The ultimate optimal standard has not been agreed upon by all authors in the literature; nonetheless, numerous articles modify particular aspects of these standards [8].
The purpose of this work to study the effect of changing the testing parameters on the results of the volatile content, ash content and consequently on the correct judgment of the quality of charcoal samples. The other purpose is to find an empirical formula for judgment the quality of charcoal based on the chemical properties of charcoal samples (heat of combustion, fixed carbon content & volatile matter content) ended by classes and quality index.
Palm tree waste charcoal, (DPT) Coconut charcoal, Dragon (sawdust charcoal), Charcoal made from natural products and Charcoal made from unknown raw materials are the five most often used types of briquetted charcoal in Saudi Arabia. A two-hour drying process at 60 degrees Celsius was used to decrease high moisture from each kilogram of charcoal. Following that, at room temperature, polyethylene bags with fine powder of charcoal were packed. Using a Raymond mill (Marconi, model: WD-CG1), the particle size range of the charcoal was independently reduced, while considering the specifications of the relevant standards.
Proximate analysis
Finding the concentrations of fixed carbon, volatile matter and ash content is a regular step in the proximal analysis process (Table 1) below provides a breakdown of the stages a specimen must take to get to this stage.
| Characteristics | EN 1860-2 | DIN 51749 |
|---|---|---|
| Fixed carbon | shall be minimum of 75% by mass | ≥ 80 % |
| Ash content | shall not exceed 8% on dry basis | ≤ 4 % |
| Moisture content | shall not exceed 8% | ≤ 8 % |
Volatile Matter (VM)
Three separate standards, ASTM D1762 and ISO 562, were used to describe the treatment conditions. Specifically, Deer Brand Charcoal, DPT Coconut Charcoal, Palm Tree Waste Charcoal made from natural products and Charcoal made from unknown raw materials were the five unique samples used in this experimental research. The study's main area of attention was volatile matter. The samples were dried at a temperature of 105 °C (with a fluctuation tolerance of 2 °C) until they attained a constant mass, which was then used to determine the amount of volatile material present.
With options for both horizontal and vertical entrances, a muffle furnace (Jung brand, model 0212) was employed. One gram of the sample material was utilized per test and crucibles with a lid were used. These crucibles were pre-calcined at 700 degrees Celsius for thirty minutes. Strict adherence to the multiple requirements listed in each standard was upheld throughout the whole analysis procedure. As shown in (Table 2), this consisted of the following adjusting temperature and placing samples within the apparatus. Time and various granulometry patterns.
| Treatments | Specification | Granulometry (mesh) | Temp. (°C) | Time (min) |
|---|---|---|---|---|
| ASTM D1762 | Charcoal | 0<x ≤ 20 | 950 ± 0.5 | (1) 2, (2) 3, (3) 6 |
| ISO 562 | Coal and Coke | 0<x ≤ 70 | 900 ± 5 | (3) 7 |
All standards remove moisture from the product at temperatures near to the boiling temperature of water to calculate the final volatile matter concentration. As a result, the next formula has been selected to harmonize the many formulations into a single equation:

Where:
M1: Masse of dry solid fuel (g) before heating;
M3: Masse of residues (g) after heating at 900 °C and 950 °C.
Ash content
The residue that is left over after solid fuel has burned is referred to as ash under certain circumstances (ASTM D1762, 2007). Depending on the quantity of unprocessed iron oxide (typically darker than cement) present, it appears as a tactile powder with a color range ranging from grey to black. Magnesium Oxide (MgO), Iron Oxides (FeO, Fe2O3), Silica (SiO2), Calcium Oxide (CaO) and alumina (Al2O3), make up the majority of the constituents of ash [9]. The treatment methods complied with the requirements set out in ASTM D3174 and ASTM D1762 norms. The particular specifications of the selected standards were strictly adhered to at every step of the analysis, covering a variety of factors such as granulometry, temperature and time patterns, as shown in (Table 3). Every test involves a series of operations involving 3 crucibles. 1 gram of dry fuel is then added after they have first been dried, their weights having been noted. The entire set-up is then placed inside a muffle furnace, where it is subjected to various temperatures and exposure times as per the treatment parameters described in the standards. The crucible is taken out after the anticipated temperature decrease, chilled in desiccators and then weighed with the deposit to determine the test's reliability. The following formulas are used to determine the various parameters following experimental testing:
Ash:

where
M2: Ash mass (masse of the residue after incineration at 750 °C (g).
M1: Masse of the dry sample (g).
Fixed Carbon:
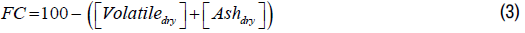
| Treatments | Specification | Granulometry (mesh) | Temp (°C) | Time (hrs.) |
|---|---|---|---|---|
| ASTM D1762* | Charcoal | 0<x ≤ 20 | 750 | 6 |
| ASTM D3174** | Coal and Coke | 0<x ≤ 60 | 700-750 | 4 |
Calorific value
An adiabatic bomb calorimeter is used in a laboratory to measure the calorific value. A unit of sample mass is contained within this equipment in a crucible and the entire assembly is then put inside the bomb calorimeter. Here, specified combustion conditions are applied to the sample following the procedures in (ASTM D2015-96, 1998). The sample is placed into the crucible holder before being loaded into the weighed crucible. The vessel body is lined up with the lid assembly and the cap is firmly fixed up until it touches the lid's top. The vessel was filled with oxygen to a pressure of 3000 kPa after being set in the vessel holder located beneath the filling station. The vessel then goes inside the measurement chamber and the lid is tightly fastened. Use the START button to start the process and get the heating value.
Moisture content
The results presented in (Figure 1) indicate that the moisture content varied depending on the test methods used, which differ in the duration of drying the samples at 105 °C. This suggests that a one-hour period at 105 °C may not be sufficient to obtain accurate moisture content measurements and a longer drying time is necessary. It was noted that moisture loss stabilized after 2 hours.
Volatile matter
The ASTM D1762 (2013) reference clearly showed the high value, while the ISO 562 standard produced lower values for volatile matter for the same product (Figure 2). The results highlight the diversity in detecting volatile materials, both across different standards. It is advised to use the ASTM D1762 standard since it is the most suitable when working with newly produced lignocellulosic materials that have not experienced pyrolysis. Verifying this information is crucial because there have been cases in the literature [10] when these recommendations were not followed. As a result, gathering data on volatile matter from various sources without standardizing the procedures can result in unreliable findings. Several publications aggregate and classify volatile material content in the literature without taking into account the best standard [11].
Lower volatile matter suggests that the briquettes may be more difficult to ignite but will burn smoothly once they are, whereas higher volatile matter produces higher combustibility at lower ash contents [12]. The highest permissible VM levels for briquettes made from palm waste are 21.33 (± 0.21), 23.91 (± 0.19) respectively, according to ISO 562 and ASTM D1762. The lowest VM values, on the other hand, for a sample of Deer brand charcoal vary from 5.93 to 6.47. The low extractive content and high lignin concentration of wood may contribute to the less volatile nature of charcoal. Increased amounts of volatile matter do not always translate into decreased burning efficiency. Flammable gas, when included in the composition, increases the burning capacity by correspondingly lengthening the flame and hastening coal ignition. On the other hand, the ability to burn can be reduced if nonflammable gas is present.
Ash content, fixed carbon and calorific value
It has been noted that different approaches can produce results that are noticeably different when attempting to determine the volatile matter and ash content in charcoal. Depending on the country of reference, the recommended heating temperature fluctuates between 875 °C and 1040 °C and the recommended heating time ranges from 7 to 20 minutes. Temperatures between 700 °C and above 850 °C are defined for ash measurement methods, with a prospective international norm of 815 °C being considered, beyond the range of the typical American range of 700-750 °C. Moreover, there is no agreement on the ideal rate of heating for detecting ash in coal samples that are abundant in calcite and pyrite (Table 4) displays the results of evaluating the ash content, fixed carbon content and calorific value of five charcoal samples obtained from the Saudi market using two different methodologies.
| Fuels | Treatments | Ash (%) | Fixed Carbon (%) | Calorific Value (KJ/Kg) |
|---|---|---|---|---|
| Palm Tree Waste | ASTM D1762 | 9.6(± 0.2) | 64.5 (± 0.1) | 24498.3 |
| ASTM D3174 | 10.8 (± 0.1) | 65.3 (± 0.1) | ||
| Deer Brand Charcoal | ASTM D1762 | 2.9 (± 0.2) | 89.3 (± 0.1) | 31403.9 |
| ASTM D3174 | 3.8 (± 0.1) | 89.9 (± 0.1) | ||
| Coconut Sawdust | ASTM D1762 | 2.4 (± 0.1) | 80.9 (± 0.1) | 29242.2 |
| ASTM D3174 | 2.8 (± 0.1) | 81.4 (± 0.1) | ||
| Charcoal made from natural products | ASTM D1762 | 2.66 (± 0.1) | 86.89(± 0.1) | 30638.3 |
| ASTM D3174 | 2.69 (± 0.1) | 86.92(± 0.1) | ||
| Charcoal made from unknown raw materials (Indonesian product) | ASTM D1762 | 2.4 (± 0.1) | 89.39(± 0.1) | 31557.2 |
| ASTM D3174 | 2.5 (± 0.1) | 89.49(± 0.1) |
The ASTM D3174 method’s ash content values were slightly higher than those obtained using the ASTM D1762 method. However, Table 3 shows that Indonesian product and Deer Brand Charcoal have the highest Performance-Based Standards (as shown by the average higher Calorific Values). This information is consistent with that given by Goudeau JC [13], who stated that "coal (whatever its nature) has the highest PCI (average lower C.V) of solid biomass and which is generally between 26,000 and 34,700 kJ/kg [14]”. The palm tree waste biomass composition contains clay thus it is weaker than the other samples. In general, the results are deemed satisfactory as their values are greater than the lowest Performance-Based Standards (PCS) values recommended by the German and Austrian and standards for briquette fuels (Germany DIN 51,731/DIN plus, Calorific value 17,500-19,500 kJ/kg, Austria ÖNORM M7135, Calorific value ≥ 18,000 kJ/kg) [15-18] (Table 5).
| Characteristics | SASO-GSO-2583:2021 |
|---|---|
| Carbon content (fixed carbon) Lump type | Minimum of 75% by mass |
| Carbon content (fixed carbon) Type briquette | Minimum of 60% by mass |
| Ash content -Lump type | Shall not exceed 5% |
| Ash content-briquette type | Shall not exceed 13% |
| Moisture content | Shall not exceed 10% |
| Volatiles matter. (lump) | Shall not exceed %20 |
| Volatiles matter(briquette) | Shall not exceed %27 |
The graph depicting the regression equation in (Figure 3) illustrates a very strong linear correlation between the percentage of fixed carbon and the heat combustion with regression value of R2=0.9986 (nearing 1.0). This indicates a very strong positive correlation between the two variables. An increase in the value of fixed carbon in charcoal is typically accompanied by a corresponding increase in its heat combustion (Figure 4). Illustrates the inverse relationship between the average percentages of ash content and heat combustion for each charcoal briquette sample. Lower ash content is associated with a higher heating value for briquettes, while higher ash content can lead to dust emissions that contribute to air pollution. Consequently, higher ash content can lower the calorific value, impacting combustion volume and efficiency (Figure 5). indicates an inverse relationship between the calorific value and the percentages of volatile materials in the samples. As the percentage of volatile materials increases, the calorific value decreases. Additionally, it was observed that the decrease in calorific value was minimal until the percentage of volatile materials exceeded 15%, at which point there was a significant decrease [19-24].
Figure 4. The relationship between the average % of ash content and heat combustion for each charcoal briquette sample. Illustrates the inverse relationship between the average percentages of ash content and heat combustion for each charcoal briquette sample. Lower ash content is associated with a higher heating value for briquettes, while higher ash content can lead to dust emissions that contribute to air pollution. Consequently, higher ash content can lower the calorific value, impacting combustion volume and efficiency.
Figure 5. The relationship between the average % of volatile matter and heat of combustion for samples indicates an inverse relationship between the calorific value and the percentages of volatile materials in the samples. As the percentage of volatile materials increases, the calorific value decreases. Additionally, it was observed that the decrease in calorific value was minimal until the percentage of volatile materials exceeded 15%, at which point there was a significant decrease.
Quality index calculation and new proposed classes for charcoal products
Most of standards all over the world deals with the specification of charcoal have stated certain requirements to judge the quality of charcoal. These standards took into account the moisture content, ash content and fixed carbon as the most important chemical properties of charcoal which give an indication about the quality of the products [25]. One of the most important properties which govern also the quality of charcoal is the heat of combustion or the calorific value. Unfortunately, all of the available standards do not take into account when judgment the quality of charcoals the heat of combustion (Table 6) shows the characteristic limits of charcoal according to the requirements of EN 1860-2 and DIN 51749 [26-30].
| Characteristics | Limits |
|---|---|
| Fixed carbon | ≥ 80 % |
| Ash content | ≤ 4 % |
| Moisture content | ≤ 8 % |
| Volatile matter content % | ≤ 18 |
| Heat of combustion | ≥ 24 MJ/Kg |
The proposed limits and quality index
The limits for acceptance of the quality of charcoal samples could be summarized in (Table 7) which includes the limits for ash content, fixed carbon, moisture content, volatile matter content and heat of combustion [31-33].
| Class | Quality index value |
|---|---|
| 1 | ≥ 1 |
| 2 | 0.8- less than 1 |
Depending on the approach used, there can be variations in the percent of volatile matter and ash content; each standard has unique qualities that can affect the outcomes. To compare the ash content and volatile matter in a substance, it is critical to standardize the procedure, as indicated in the literature. For volatile matter, the ISO 18123 (2015) standard revealed the highest results, while the ASTM D1762 (2013) standard clearly displayed the lowest value. By using various international standards related to ash testing, it was found that there is a large difference in the present of ash content, fixed carbon and calorific values when monitoring the results.
It is observed that Deer brand charcoal had the greatest fixed carbon, with a fixed carbon content of 89.3%, the least volatile matter, with a volatile matter level of 5.93% and the least ash, with a concentration of 3.8%, according to the obtained results using the ASTM D1762 method. While these characteristics changed when using the ASTM D3174 standard, where the ash content of the same sample decreased by 23.68% and thus the amount of fixed carbon increased slightly inversely by 0.7%. According to the study's findings, charcoal made from Deer Brand Charcoal and Charcoal made from natural products (Indonesian product) may be the ideal choice for cooking fuel in homes and restaurants due to their better fuel properties. This study can be contributing to determining the best specifications, standards, requirements and limitations that can be applied to these products to ensure the highest quality.
All data underlying the results are available as part of the article and no additional source data are required.
The authors sincerely appreciate the support of SASO for providing access to the lab, testing materials and charcoal samples. Special thanks to Sami Alsaeed and Dhafir AlAjmi for technical assistance.
Regarding the release of this paper, there are no conflict of interest according to the authors.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Journal of Environmental Hazards received 51 citations as per Google Scholar report