Research - (2021) Volume 9, Issue 9
Received: 02-Nov-2021
Published:
23-Nov-2021
Citation: Chahkandi, Benyamin, Mohammad Gheibi and
Amir Takhtravan. "Prioritizing Decomposition Methods for Measuring Small Amounts of Bismuth Using TOPSIS Scoring Technique Considering Economic, Environmental and Operational Indicators". J Glob Econ 9 (2021): 389.
Copyright: © 2021 Chahkandi B, et al. This is an open-access article distributed
under the terms of the creative commons attribution license which permits
unrestricted use, distribution and reproduction in any medium, provided the
original author and source are credited.
Due to the wide application of bismuth in various industries and the environmental effects of its release in water and soil resources, the necessity of measuring its small amounts becomes apparent. Various decomposition methods such as electrochemical systems, molecular spectrophotometry, atomic absorption spectroscopy and ICP can be used to measure bismuth. In this study, 18 analytical measurement methods were evaluated in terms of economic, environmental, feasibility, measurement speed and accuracy. In this study, the Technique for Older-Preference by Similarity to Ideal Solution (TOPSIS) decision-making method was employed to analyze and prioritize different options. The results showed the implementation of molecular spectrophotometric systems such as color change, methylene blue-bismuth and ionic complex of iodide-bismuth absorption and atomic absorption spectroscopic methods in terms of economic, environmental and operational indicators score higher than other methods and are the basis of industrial selection and environmental assessments.
Bismuth • Decomposition methods • Economic indicators • Environmental indicators • TOPSIS
Bismuth is a chemical element with the symbol Bi and atomic number 83 and atomic mass 208.98. It has a density of 10.05 grams per cubic centimeter and its melting and boiling points are 271 and 1564 degrees Celsius, respectively. Bismuthinite and bismite are the most important bismuth ores. Bismuth is a weak trivalent, white crystalline, heavy and brittle with a slight pink tinge, and is chemically similar to arsenic and antimony [1]. Bismuth has been known to mankind since ancient times but was often confused with lead and tin until 1753 when scientists could distinguish them. The origin of the word bismuth is not clear. It may be derived from the Arabic word (similar to kohl stone) or the German words "Weisse Masse" and "Wismuth" meaning white mass, which became the Latin, word Bisemutum in the 16th century. Bismuth is the most magnetic of all metals and has less thermal conductivity than all elements except mercury. Bismuth has unusually low toxicity compared to other heavy metals, and its alloys have been used as a substitute for lead since it is toxic to humans. However, several human and animal toxic effects of bismuth compounds have been reported. Recent studies have shown that excessive amounts of bismuth compounds affect the nervous system which convinced scientists to suggest toxicity of bismuth [2]. In recent years, bismuth has received much attention due to its chemical and physical properties. First, bismuth compounds became important in medicine. In 1786, bismuth was used to treat indigestion. In 1889, it was discovered that bismuth could be useful as an anti-syphilis agent. Its medicinal uses then spread to topical antacids and creams for the skin. Bismuth compounds are used in the manufacture of cosmetics, pigments and medicinal applications. It is also used in metallurgy, cast iron, electrochemistry, plastics, paints, oils and printing inks. The presence of small amounts of bismuth could have many effects on the magnetic, mechanical and physical properties of some materials. Bismuth improves the stability of iron carbide. The presence of small amounts of bismuth reduces the strength and brittleness of some alloys. Therefore, measuring small amounts of bismuth in different industries is of great importance. However, various methods have been proposed to detect and measure small amounts of bismuth. This research intends to use the TOPSIS decision-making system to evaluate different methods of measuring bismuth in terms of economic parameters, environment, feasibility, measurement speed and accuracy [3].
Bismuth measuring methods
There are several methods for measuring small amounts of bismuth, including electrochemical methods, molecular spectrophotometry, atomic absorption spectrometry, and Induced Coupled Plasma Spectrometry (ICP). Some of these methods are evaluated in this research, which is elaborated in Table 1.
TOPSIS prioritization system
The TOPSIS method was first proposed by Hwang and Yoon in 1981. This method is based on considering the distance of an option from the ideal and counter-ideal points. That is, the selected option should have the shortest distance from the ideal and at the same time farthest from the counter-ideal. Only when the indicators and their relative weights are definite numerical values, the TOPSIS method is applicable [4].
In this method, first after making the decision matrix scale less and assuming the weight vector of the indicators (W), the scale less weight matrix is calculated using equation (1).

In this equation, W is the diagonal matrix of the weight of the indicators whose only main elements are non-zero, ND is the decision matrix and V is the scale less weight matrix. Positive and negative ideal solutions are then calculated, based on Equation (2).
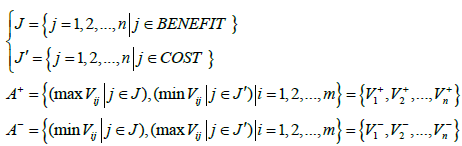
The relative proximity calculation i A to the ideal solution in which di+ and di− are the amount of the distance is then calculated using Equation (3).
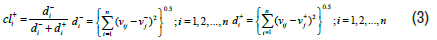
The closer the option size is to the ideal solution , the closer the value cli+ is to the unit. The above relationships are programmed and used in the MATLAB 2013b coding environment.
All the options mentioned in Table 1 are evaluated based on 5 economic, environmental, feasibility, measurement speed and accuracy indicators then have been balanced in a range of 0 to 20 using mathematical proportions which are presented in Figure 1. Also, according to importance, each of the five indicators was weighed. These weights are 0.25, 0.2, 0.1, 0.15 and 0.3 for economic, environmental, feasibility, measurement speed and accuracy indicators respectively.
| Code | Measurement system | Details |
|---|---|---|
| A1 | Electrochemical | In this method, kappferon ligand is used to form a bismuth complex and the measurement takes place through naked voltammetric method. |
| A2 | Electrochemical | Ion-selective electrode along with polymer membrane was used to measure small amounts of bismuth. |
| A3 | Electrochemical | In this method, bismuth sulfide nanoparticles were dispersed in polyvinyl chloride membrane and used to measure bismuth ion. |
| A4 | Electrochemical | In this method, bismuth is complexed with Bermo pyrogallol-red ligand and carbon is accumulated on the surface of the paste electrode. |
| A5 | Molecular spectrophotometry | In this method, bismuth ion is adsorbed on the surface of activated carbon after reacting with thiourea and bromide ions in an acidic environment. |
| A6 | Molecular spectrophotometry | In this method, after mixing the bismuth ion with the complexing agent of red bermogallol, it was extracted in 114-X ion micelles surfactant medium. |
| A7 | Molecular spectrophotometry | In this method, methylene blue has been used as a complexing agent with bismuth and after the formation of methylene blue-bismuth complex; the color intensity was measured at a wavelength of 548 nm. |
| A8 | Molecular spectrophotometry | In this method, after complexing bismuth and iodide ion, an ionic complex of bismuth-iodide is formed, which after reacting with methyltrioctylammonium chloride becomes non-ionic and is extracted in carbon tetrachloride solvent. |
| A9 | Atomic absorption spectrometry | In this method, thiocyanate ion was used to form an anionic bismuth complex, after reaction with the cationic steel pyridinium chloride surfactant; the complex was ionized and extracted in carbon tetrachloride extracting solvent. |
| A10 | Atomic absorption spectrometry | In this method, after mixing by di-tizone ligand in micelles Triton-114X medium bismuth ion is extracted which is diluted by tetrahydrofuran solvent and 20 μl of the solution is injected and measured into the graphite furnace atomic absorption spectroscopy. |
| A11 | Atomic absorption spectrometry | In this method, a syringe containing silica gel modified with 3-aminopropyl trimethoxysilane was used as the adsorbent. |
| A12 | Atomic absorption spectrometry | The decomposition column used is 2-mercaptobenzothiaz placed on the surface of alumina coated with sodium dodecyl sulfate, which traps bismuth ions. |
| A13 | Atomic absorption spectrometry | In this method, solid phase extraction based on octa-decyl bonded silica cartridge modified with cyanide has been used to pre-concentrate very small amounts of bismuth. |
| A14 | Atomic absorption spectrometry | In this method, chromosorb-107 solid phase was packed in a special syringe and after adsorption of analyte on the surface of solid phase, 3 mol / l nitric acid was used to desorb the analyte. |
| A15 | Atomic absorption spectrometry | An on-line preconcentration/separation system for the determination of bismuth in environmental samples by FAAS. |
| A16 | Plasma atomic emission spectrometry | On-line preconcentration system for bismuth determination in urine by flow injection hydride generation inductively coupled plasma atomic emission spectrometry. |
| A17 | ICP | In this method, after creating a complex of bismuth ion and 8-hydroxyquinoline ligand, the mentioned complex is extracted by cloud point extraction method in Michely Triton 114-X environment. |
| A18 | ICP | In this method, after the formation of bismuth-8-hydroxyquinoline complex, the sample solution is passed through the 7-XAD amberlite column and after washing the column with 20% nitric acid solution, it is transferred to a container of sodium borohydride and after revival it is is transferred to a plasma atomic emitter in order to be measured. |
Moreover, the data depicted in Figure 1 were operated as the input for the TOPSIS method equations. Each option is measured in terms of different indicators to the positive ideal or the best answer and also to the negative ideal or the worst answer. Finally, based on these distance measurements, the options are prioritized. The results of the positive and the negative ideal distances are shown in Figures 2 and 3 respectively.
As shown in Figure 2, Options A7 (methylene blue-bismuth complex) and A8 (ionic bismuth-iodide complex) have the shortest distance from the ideal positive answer, indicating their desirability.
Considering Figure 3, it can be concluded that the measurement options A7 (methylene blue-bismuth complex) and A8 (ionic bismuth-iodide complex) have the greatest distance from the negative ideal answer. Options A1 (kaferron-bismuth ligand complex) and A3 (bismuth sulfide nanoparticles and PVC membranes) have the highest distance from the positive ideal answer and the lowest distance from the negative ideal answer.
The TOPSIS analysis final results are shown in Figure 4. Options A7 and A8 with weight indices of 0.204 and 0.2 have the highest degree of importance and create the best conditions. Consequently, A7 and A8 methods are more common for measuring small amounts of bismuth in industries [5].
Comparing different methods indicates that spectrophotometric and spectroscopic measurement systems of atomic absorption have created better conditions and have better rankings in terms of economic, environmental and measurement accuracy than electrochemical and ICP methods [6].
Measuring small amounts of bismuth in industries considering two aspects including Industrial applications and epidemiological effects seems inevitable. From the epidemiological and environmental perspective, small amounts of mineral compounds accumulate in the living organisms' body which leads to destructive effects on health due to the biological magnification. In the present research, different methods and techniques of bismuth measurement were evaluated divided by four general methods including electrochemical, molecular spectrophotometry, atomic absorption spectroscopy and ICP in terms of economic, environmental, feasibility, speed and accuracy of measurement using the TOPSIS method. The results showed that the A7 (methylene blue-bismuth complex) and A8 (ionic bismuth-iodide complex) measurement methods have the highest distance from the negative ideal answer and the lowest distance from the positive ideal answer. Therefore, they were selected as the main options of the bismuth measurement system.
Journal of Global Economics received 2175 citations as per Google Scholar report