Research Article - (2022) Volume 12, Issue 4
Received: 28-Feb-2022, Manuscript No. jcde-22-55719;
Editor assigned: 02-Mar-2022, Pre QC No. P-55719;
Reviewed: 17-Mar-2022, QC No. Q-55719;
Revised: 28-Mar-2022, Manuscript No. R-55719;
Published:
05-Apr-2022
, DOI: 10.37421/2165-784X.22.12.444
Citation: Mindahun, Tayto. “Removal of Pb (II) Ions from Aqueous Solution and Industrial Wastewater using Activated Carbon Prepared from Flax Straw.” J Civil Environ Eng 12 (2022): 444
Copyright: © 2022 Mindahun T. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : None
This study aimed to prepare AC from flax straw and investigate its potential for the removal of Pb (II) ions from aqueous solution and real industrial wastewater. AC was prepared by chemical activation method using H3PO4 as activating agent. The effects of initial Pb (II) ion concentration, adsorbent dose, contact time and pH on the removal efficiency were studied by using aqueous solution prepared from Lead nitrate (Pb (NO3)2) on a batch mode. Response surface methodology was used in order to carry out experimental runs. The collected wastewater sample was characterized before and after treatment according to APHA methods. Activated carbon was characterized and results showed that the flax straw AC had 8.04% of moisture, 6.04% of ash, 18.615% of volatile matter, 79.421% of fixed carbon, 459.807 mg/g of iodine number and surface area of 489.455 m2/g. Physico-chemical characteristics showed that raw wastewater had a concentration of 3.95 mg/L Pb (II), 158.52 mg/L BOD5, 2482 mg/L COD, and 652.667 mg/L TSS. The highest removal efficiencies of Pb (II) metal ion which was achieved from aqueous solution and paint wastewater were found to be 95.16% and 78.73%, respectively. The experimental data are fitted with pseudo-second order model and adsorption of Pb (II) on flax straw AC fits the model of Langmuir very well. The results of the study suggested that flax straw AC can be used as an adsorbent for the removal of Pb (II) ion from industrial wastewaters.
Flax straw • Removal Pb (II) • Activated carbon • Optimum conditions
Currently there is a rapid population growth and there is also a growing trend of industrial sector development. Continued population growth and rapid industrialization are found to be the cause of wastewater discharge into the environment, affecting the environment, human health and the life of future generations. The liquid waste discharged from industries contains heavy metals like Pb, Cd, Cr, Cu, Ni and Zn toxic to living organisms [1]. Among these toxic metals; Lead is a potent poison and is harmful in even very small amount to humans and other living organisms because of its known toxicity [2]. Therefore, industries have to use treatment technologies in order to remove this toxic metal from wastewater. A number of advanced technologies like precipitation, ion exchange, coagulation and electro dialysis can be used to remove heavy metals from industrial effluent [3]. But; it is difficult to use some of these advanced technologies in all level of developing countries for industries to deal with heavy toxic industrial wastewater. In addition to this, these modern technologies have numerous drawbacks such as incomplete metal ion removal, high energy and reagent costs, and toxic sludge [4]. Therefore, the use of various adsorbents like activated carbon is of great interest due to environmental concerns. Adsorption technique using activated carbon look to be more attractive due to its simplicity, ease of use, high efficiency, and being economical in the removal of heavy metals from wastewater [5-7]. Despite the fact that activated carbon has numerous applications in various industries, the main challenge is obtaining the best and excess raw materials with the potential to produce effective activated carbon since commercial activated carbon is primarily coal-based activated carbon, which is more expensive. Consequently, it is critical to look for alternative raw materials from lignocellulose agricultural waste since they are renewable supply, low cost, easy availability, and environmental friendliness [8].
Conversion of lignocellulose agricultural waste to activated carbon is a possible and feasible approach. It can be done via physical activation or chemical activation method [7]. Chemical activation methods often carried out at lower activation temperatures and shorter activation time than physical activation. Chemical activation produces higher yields of activated carbons than physical activation because the chemical agents utilized have dehydrogenation capabilities, which prevent the creation of tar and limit the development of other volatile compounds [9].
Several researchers have adopted various low-cost adsorbents, but there is still a need to develop activated carbon from cheaper and readily available materials, which can be effective and economical for the removal of heavy metals from wastewater. It is known that flax straw is one of the low cost agricultural waste by-products [10]. Flax straw contains high carbon and volatile matter content and low ash percentage [11]. So, it is possible to convert flax straw to AC. And also, flax straw is availability most regions of Ethiopia since it is preferable at low soil fertility. However, flax straw is not used for animal feed rather it is directly discharge to the environment and pollutes the environment. Thus, using this biomass for production AC is very important. Phosphoric acid activation causes less environmental and toxicological contamination. considering this, this study was used H3PO4 as activating agent for the preparation of AC from flax straw for lead metal ions removal from aqueous solution and real industrial wastewater.
Preparation of activated carbon
Flax straw was first collected from Holeta Agricultural Research Center, Ethiopia. Then, the straw was chopped into small pieces, washed and sun dried. The sun dried sample was milled to a size of 0.5 mm using cutting mill (Fritsch Cutting Mills Pulverisette, Germany). The sample was dried in oven (PRI/1501A, India) at 105°C for 24 h and 11 g of sample was impregnated with 30.3 ml of solution (30%, 50% and 70% H3PO4) at impregnation ratio of 2.75 for 24 h. After that, it was dried in oven at 105°C for 24 h and carbonized in a muffle furnace (MV 106, Germany) at carbonization temperatures of 400, 500 and 600°C at carbonization times of 30, 60 and 90 min [8]. The sample was washed with distilled water, 0.5 M HCl (37%) and 0.5 M NaOH until the effluent water shows neutral pH after cooling in desiccator. Lastly, sample was dried in an oven at 105°C for 24hrs, grinded with mortar and pestle, sieved using 125 μm sieves and stored in plastic bottles for further analysis.
Characterization OF activated carbon
Proximate analysis of the activated carbon (Ash content, moisture content and volatile matter were determined by ASTM D2866-94 (2004), ASTM D2867- 94 (2004) and ASTM D5832-98 (2014) respectively. whereas, fixed carbon content was determined by was subtracting the resultant of summation of percentage moisture, ash, and volatile matter subtracted from 100 [12]. Iodine number was measured by ASTM D4607-94 (2006). The surface area of flax straw activated carbon was determined by BET surface area analyzer (SA- 9600 Series Surface Area Analyzer, Horiba instruments, Inc.).
The surface morphology of the flax straw activated carbon sample was analyzed by the Scanning Electron microscope (Model: FEI-INSPECT-F50). To identify the functional groups that might be involved in the binding of heavy metal ions on its surface, activated carbon sample was analyzed by Thermo Scientific FT-IR instrument (Model: SMART iTX, Thermo scientific). The XRD analysis was carried out by using X-Ray diffractometer (model: XRD-7000 X-RAY Difractometre, MAXlma) under the condition of voltage 40.0 KV and current 30.0 mA. The scan was obtained from a range of 5.000 to 85.000 (Bragg angle 2θ) of sampling pitch 0.02° every 0.40 (sec).
Batch adsorption experiments
Preparation of synthetic wastewater: The lead (II) stock solution was prepared by the methods of [13] with little modifications. Synthetic wastewater samples of 500 mg/l was prepared by dissolving 0.8 g of lead nitrate ((99% New Delhi, India) in 500 ml distilled water. The lead standard working solutions were prepared by serial dilution using equation 2.1 given below.
C1V1 = C2V2 (2.1)
Where: C1 is the initial concentration (mg/l), C2 is the final concentration (mg/l), V1 is the initial volume (ml), and V2 is the final volume (ml)
Calibration curve plot for lead metal solution: The calibration curve of absorbance against lead metal concentration was obtained by the method of [14]. The concentrations of lead metal ions in solutions were estimated by measuring absorbance at wavelength of 300 nm by UV-Vis Spectrophotometer (Model-UVD-3200, LABOMED, INC). Finally linear relationship of absorbance vs. concentration of lead was plotted and final concentration of lead was found from the equation 2.2.
Absorbance = slope * Ce +Y - intercept (2.2)
Batch experiment procedures
The batch experiments were performed in accordance with the procedure established by [4] with little modifications. In this study, for each experimental run, working solutions (40-80ppm) were obtained from the stock solution by serial dilution and the pH was adjusted from (2-10) by adding 0.1M HCI or 0.1M NaOH. Then, adsorbent dose (1- 4) g was added in each 250ml Erlenmeyer flask containing the solution and samples were shaken on incubator shake (Model: Excella E24R) at room temperature, at a constant rate (250 rpm) at contact time of (10-120) minutes. The samples were collected at the end of time required for adsorptions. The supernatant liquid was filtered by qualitative filter paper of seize 15 cm and collected 100 ml volumetric flask and analyzed for lead metal ions removal percentage using UV-Vis Spectrophotometer (UVD-3200, LABOMED, INC.) at 300nm. The percent removal of metal ions was calculated by using the following formula [4]:
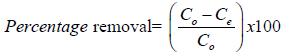 (2.3)
(2.3)
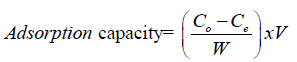 (2.4)
(2.4)
Where,
Co (mg/l) is the initial concentration of lead metal ions and, Ce is the concentration of lead metal ions at equilibrium, V (L) is the volume of lead metal ions solution in the flask and W (g) is the mass of activated carbon used in the experiment.
Isotherm study
Isotherm study was performed according to the procedures developed by Mustapha S, et al. [15] with little modifications. Adsorption isotherms were investigated for (20–90) mg/l initial lead metal ion concentrations using 4 g of flax straw activated carbon sample added to 100 ml of the metal ion concentrations and shaken for equilibrium time of 101 minutes at 250 rpm. Two isotherm models, namely Freundlich and Langmuir, were employed in this study.
Kinetic study
Kinetic study was conducted according to the methods developed by Mustapha S, et al. [15] with little modification. Kinetics study was conducted by taking 100 ml of lead metal ions solution with initial concentration of 46.7 mg/l in 250 ml Erlenmeyer flasks and adjusting the pH to 4.9. Then 4 g of flax straw activated carbon was added to each samples and the solution will be agitated at 250 rpm in incubator shaker at different contact time(10-80) minutes at room temperature. The kinetics of lead metal ions adsorption on flax straw activated carbon was analyzed using pseudo first-order and pseudo second-order kinetic models.
Experimental design
Initial Pb (II) ion concentration, adsorbent dosage, contact time and pH on the removal efficiency of Pb (II) ion using flax straw activated carbon over three levels were analyzed by Box–Behnken methods in order to understand their effect on adsorption of lead metal on AC. Removal efficiency was considered as response variable during the experiment.
Collection and characterization of industrial wastewater
Plastic bottles of 2000 ml was used to collect wastewater samples from KADISCO paint factory located in Addis Ababa, the sub city of Akaki Kality after cleaning with tap water, hydrochloric acid and distilled water and transported to the laboratory. Lead metal ions concentration and physicochemical characteristics of the wastewater sample (temperature, pH, and turbidity, BOD5, COD, DO, TSS and TDS) were analyzed before and after adsorption according to APHA (2017) method.
Characterization of flax straw activated carbon
The physicochemical properties of the prepared activated carbon were determined and the results are presented in Table 1.
| Independent Variables | Unit | Levels | |
|---|---|---|---|
| Minimum | Maximum | ||
| Adsorbent dose | g | 1 | 4 |
| pH of the solution | - | 2 | 10 |
| Initial lead metal concentration Contact time |
Mg/l minutes |
40 10 |
100 120 |
As it indicated in the Table 1 above, physicochemical properties of the prepared activated carbon were in a reasonable agreement with standards set by Maulina S and Iriansyah M [16]. The presence of low ash content in activated carbon resulted in high fixed carbon content. This is because low ash percentage increases the solid yield of the carbon and produce high fixed carbon. According to the data obtained from Ukanwa K, et al. [17], activated carbon derived from chemically activated agricultural wastes has a surface area ranging from 200 m2/g to 2000 m2/g. As a result, the result of this study is in reasonable agreement for its surface area. To sum up, activated carbon having high surface area would have better adsorption potential in heavy metal removing process.
Fourier transformer infrared spectroscopy (FTIR) analysis
FTIR spectroscopy was used to conduct a quantitative investigation of the key functional groups involved in the adsorption of lead metal ions onto activated flax straw. In the range of 4000 cm-1 to 400 cm-1, the FTIR spectra of lead metal ions loaded and unloaded activated carbon treated with phosphoric acid were observed. The recorded adsorption spectra before and after adsorption are shown in Figure 1.
Because the Pb (II) ion binds to the activated carbon's active sites, the FTIR spectrum of the activated carbon exhibits variances in peak frequencies, showing the presence of ionizable functional groups on the activated carbon that might interact with additional cat ions.
X-Ray diffraction analysis
The key objective of X-ray diffraction is to gather structural information on activated carbon crystalline solids [18]. Activated carbons can be categorized into two categories depending on their graphitizing capacity, on the basis of XRD studies [19]. Due to the formation of strong cross-linking between adjoining randomly oriented elementary crystallites, non-graphitizing carbons are hard and have a well-developed micro porosity structure. Graphitizing carbons, on the other hand, showed poor cross-linking and a less developed porous structure. The X-ray diffraction patterns of flax straw activated carbon prepared at optimal conditions are shown in Figure 2 below. The broad peak in the 2Ɵ range (20.67-30.47) O suggested that the main structures of prepared activated carbons are amorphous as expected which is a desirable feature for porous adsorbents with well-defined pores. Additionally, the lack of a distinct peak indicates that the majority of the activated carbon is amorphous, which is a favorable adsorbent characteristic [18]. This result was similar with previous studies on activated carbon produced from agricultural wastes [20,21].
Scanning electron microscopy
The main purpose of performing Scanning Electron Microscopy (SEM) technique was in order to observe the surface physical morphology of the flax straw activated carbon prepared by phosphoric acid as activating agent. Figure 3 show the SEM image with magnification of 50 μm.
Porosity is seen on the surfaces of the prepared activated carbons caused by the activating agent (phosphoric acid) evaporating during the carbonization of the sample in a muffle furnace, leaving empty spaces. Porosity is a great adsorbent texture because it gives a lot of surface area for the adsorption process [22].
Effects of individual factors on Pb (II) ion removal efficiency
Effects of pH: At low pH values, there is excessive protonation of the flax straw activated carbon surface thereby preventing the metal ions from approaching the binding sites of the surface resulting in a low removal efficiency of Pb (II) ions as it indicated in Figure 4A. This is consistent with the results obtained by Boudrahem F, et al. [23]. When the pH value is increased from 3 to 3.66, the number of H+ ions decrease and the surface of the activated carbon is negatively charged, therefore, the metal ions get the opportunity to be adsorbed on the surface of activated carbon and, as a result of this, the removal efficiency increased from 63.2 to 65.6%. The decrease in competition between protons (H+) and positively charged metal ions at the surface sites can explain the increase in metal removal when pH rises [7,14]. Further increase of pH value above 4.53 causes the removal efficiency to decrease since at high pH, there are more hydroxyl ions (OH–) in the solution which react with the Pb ions to form their insoluble hydroxides. Thus, precipitation takes place and it will clog the active site of the carbon which reduces the rate of adsorption [24].
Effect of adsorbent dose: Adsorbent dosage is another important parameter because it determines the capacity of the adsorbent for a given Lead metal ions concentration and also determines the sorbent– sorbate equilibrium of the system. The removal of Pb (II) ions was increased while the adsorbent dose was increased from 1 to 4 g, as shown in Figure 4B since increasing the adsorbent dosage results in a larger adsorption surface area [23]. A similar result was reported in the elimination of Pb (II) ion from paint industry wastewater using Activated Carbon Derived from African Arrowroot (Canna indica) Stem [7]. Furthermore, the study that was done previously by Wamb EW, et al. [25], show that in the initial stages, the amount of lead adsorbed increased linearly with respect to the adsorbent mass showing that the increase in mass of adsorbent increased the amount of available adsorbent sites at which Pb (II) ions could adsorb.
Effects of initial Pb (II) ions concentration: It is known that the initial concentration provides a good driving force to overcome all mass transfer resistance of lead ions between the aqueous solution and solid phase of activated carbon. In this study, with the increase in concentration from 40.3 to 61.1 mg/l, the removal of Pb (II) ion was found to be small as it indicated in Figure 5A. This is due to the reason that at low concentrations, the number of lead ions available in the solution is less compared to the available binding sites on the surface of activated carbon. As a result, all of the lead ions have the potential to interact with the active site [24,26].
In contrast, higher initial concentrations resulted in more lead ions being linked to the adsorbent surface, causing the active site to become insufficient and adsorbent saturation, resulting in a drop in removal efficiency [24].
Effect of contact time: Contact time is a very important parameter in adsorption processes. It determines the equilibrium time of the adsorption process. The characteristics of activated carbon and its available adsorption sites affects the time needed to reach equilibrium [26]. As it indicates in Figure 5B, there was an increase in percentage removal of lead metal ions as contact time increased from 10 to 101 minutes until equilibrium was reached. Because there were more unoccupied spaces on the surface of the activated carbon at the beginning, and metal ion uptake was higher, there was a continuous increase in adsorption capacity by expanding the time slot from the start (10 minutes in this case) to 101 minutes. However, further increases contact time beyond 101 minutes would not be sufficient to create a sufficient change in metal adsorption because unoccupied areas have already been filled, and equilibrium has been reached [27]. Apparently, the adsorption of most metal ions by activated carbon generally reaches equilibrium within 120 min [26]. Therefore, based on the result of this study 101 minutes was found to be the equilibrium time for other experiments.
Interaction effect of adsorption process parameters: As shown in the Figure 6A, increase in the contact time provided more Pb (II) adsorbed on its surface of the adsorbent. And also, as the pH of the solution increases, the adsorption efficiency of flax straw activated carbon increases. Thus, maximum adsorption occurs at around 101 minutes and pH of 4.9.
The combined effects of adsorbent dose and initial concentration on the adsorption efficiency of Pb (II) are depicted in Figure 6B as a 3D plot. Increase in the adsorbent dose provided more surface area or increased available adsorptions sites which increased the amount of Pb (II) adsorbed on its surface. As shown in the Figure 6C, the removal efficiency of activated carbon was low at maximum values of contact time and initial lead metal concentration.
Adsorption isotherm study
Figure 7 below shows Langmuir (A) and Freuindlich Plot of lead metal ions adsorption at room temperature, pH of 4.9, activated carbon of dosage of 4 g and contact time of 101 minutes and for different initial lead metal solution concentrations and Table 2 shows the parameters of these isotherm models.
| Parameters | Result |
|---|---|
| Ash content (%) | 6.04 |
| Moisture (%) | 8.004 |
| Fixed carbon (%) | 79.421 |
| Volatile matter (%) | 18.615 |
| Surface area (m2/g) | 489.4545 |
| Iodine number (mg/g) | 459.807 |
From the Table 3 above, the correlation coefficients (R2) of Langmuir and Freundlich isotherms models were found to be 0.9704 and 0.9587, respectively. Therefore, from this result we can say that the adsorption of Pb (II) on flax straw activated carbon fits the model of Langmuir very well.
| FTIR peaks | Band Wave Number (cm−1) | Assigned Functional Groups | Compound Class | ||
|---|---|---|---|---|---|
| Before Adsorption | After Adsorption | Shift Difference | |||
| 1 | 614.26 | 615.181 | 0.921 | Strong C-Br stretching | Halo compound |
| 2 | 1117.546 | 1110.797 | 6.749 | Strong C-O stretching | Secondary alcohol |
| 3 | 1379.818 | 1390.424 | 10.606 | Medium C-H bending | Aldehyde |
| 4 | 1633.412 | 1634.375 | 0.963 | Medium C= C stretching | Alkene |
| 5 | 2082.744 | 2081.780 | 0.964 | Strong N= C= S stretching | Isothiocyanate |
| 6 | 3439.421 | 3444.242 | 4.821 | Strong O-H stretching bond | Alcohol |
Adsorption kinetic study
Kinetic models (pseudo-first order and pseudo-second order) were investigated at optimum conditions (adsorbent dosage of 4 g, initial lead solution concentration of 46.7 mg/l and pH of 4.9). Both kinetic models were based on the assumption that the rate of occupation of adsorption sites is proportional to the number of unoccupied sites. The best fit model was selected based on the linear regression correlation coefficient (R2).
Pseudo-first order kinetic model
Figures 8 and 9 below indicate the plots pseudo-first order and pseudo second order kinetic model and Table 4 summarized the constant values for these kinetic models. According to the values of correlation coefficient, R2 as indicated in the Table 5 below, pseudo-second order model showed a higher (R2 =0.9903) value which indicate that the kinetics of adsorption of lead metal adsorption by flax straw activated carbon could be better described by pseudosecond order model.
| Isotherm Model | Parameters | Values |
|---|---|---|
| Langmuir | qm (mg/g) | 1.29 |
| KL (L/g) | 0.09 | |
| R2 | 0.9704 | |
| Freundlich | KF (mg/g) | 0.26 |
| nF | 2.727 | |
| R2 | 0.9587 |
| Kinetic Models | Pseudo-first Order Model | Pseudo-second Order Model | ||||
|---|---|---|---|---|---|---|
| Parameters | K1(min-1) | Qe (mg/g) | R2 | K2(min-1) | Qe (mg/g) | R2 |
| Values | 0.01 | 0.688 | 0.8814 | 0.087 | 0.572 | 0.9903 |
Flax straw AC performance for lead removal from real wastewater
Table 6 given below show the characteristics of untreated and treated wastewater. Except for temperature, the concentrations of most parameters in untreated wastewater exceeded the EEPA standard [28]. The concentration of Pb (II) ions, in particular, was found to be much greater than the standard, indicating that it must be removed to avoid environmental vulnerabilities. The results indicate that highly polluted effluent of the Pb (II) value was 3.95 mg/L as against the maximum limited Pb (II) which is 0.50 mg/L as stated by UNIDO EPA [28].
| Parameters | Untreated Wastewater | Treated Wastewater | Max Permissible Limit |
|---|---|---|---|
| Lead (Pb), mg/L | 3.95 | 0.84 | 0.5 |
| Turbidity, NTU | 3394 | 20.55 | 25 |
| pH | 4.5 | 6.9 | 6 - 8.5 |
| BOD5,mg/L | 158.52 | 31.97 | 50 |
| COD, mg/L | 2482 | 162.4 | 150 |
| DO, mg/L | 0.152 | 7.25 | 5 - 20 |
| TSS, mg/L | 652.667 | 145 | 50 |
| TDS, mg/L | 604.444 | 144.444 | - |
| Temperature(°C) | 20.2 | 20.1 | Ambient temperature ± 3 |
The treated wastewater characteristics were below the standard set by WHO (2017) except for COD and TSS. Hence, further treatment is needed to remove the excess COD and TSS before disposing to the environment. In this research, it was found that the removal efficiencies of Pb (II) ion from aqueous solution is 95.16% and from the paint industry wastewater it was found to be 78.73%. As the result of this research show, the performance of the flax straw activated carbon for the removal of Pb (II) ion in the paint industry wastewater was found to be much lower than the aqueous solution. The reason for this to happen could be paint industry wastewater contained different types of heavy metals like arsenic, chromium, copper, mercury, nickel, and silver, biological oxygen demand (BOD5), chemical oxygen demand (COD), total suspended solids and turbidity that affected the Pb (II) ion removal efficiency by competing one another in the flax straw activated carbon active site.
The study has demonstrated the possibility of developing low-cost activated carbon from cheap and abundantly available flax straw and it’s potential as an effective adsorbent for the removal of Lead metal ions from real industrial wastewater (paint industry). The removal efficiency of flax straw activated carbon from aqueous or synthetic solution is 95.16% and from the paint industry is 78.73%. This percentage removal of lead metal ion was achieved by employing the optimum parameters. The removal performance of the flax straw activated carbon for the removal of Pb (II) ion is lower in the paint industry wastewater than the removal in aqueous solution due to the occurrence of various types of heavy metals and pollutants. To sum up, this research showed that, the adsorption process using flax straw activated carbon were inexpensive, environmental friendly and has potential to adsorb pollutants and toxic heavy metals from both synthetic solution and real industrial wastewater. Lead metal ion removal was the main interest in this research; therefore, future research can focus on other metals removal by flax straw AC from wastewater.
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Google Scholar, Crossref, Indexed at
Journal of Civil and Environmental Engineering received 1798 citations as per Google Scholar report