Research Article - (2024) Volume 15, Issue 3
Received: 01-Jun-2024, Manuscript No. CSJ-24-69043;
Editor assigned: 02-Jun-2024, Pre QC No. P-69043;
Reviewed: 18-Jun-2024, QC No. Q-69043;
Revised: 24-Jun-2024, Manuscript No. R-69043;
Published:
30-Jun-2024
, DOI: 10.37421/2150-3494.2024.15.3.304
Citation: Sujith, Chudamani B and Subhas S Karki. "Synthesis of Novel Levamisole Derivatives for Their Anticancer and Antiviral Activity." Chem Sci J 15 (2024): 304.
Copyright: © 2024 Sujith, et al. This is an open-access article distributed under the terms of the creative commons attribution license which permits unrestricted
use, distribution and reproduction in any medium, provided the original author and source are credited.
All the compounds (CH-69 to CH-84) were evaluated for their cytostatic activity against human HeLa cervix carcinoma cells, human CEM CD4þ T-lymphocytes as well as murine L1210 cells. All assays were performed in 96 well microtiter plates. To each well were added (5-7.5) × 104 tumor cells and a given amount of the test compound. The cells were allowed to proliferate for 48 h (murine leukemia L1210 cells) or 72 h (human lymphocytic CEM and human cervix carcinoma HeLa cells) at 37°C in a humidified CO2 controlled atmosphere. At the end of the incubation period, the cells were counted in a coulter counter. The IC50 (50% inhibitory concentration) was defined as the concentration of the compound that inhibited cell proliferation by 50%. The cytotoxicity and antiviral activity of a new series of 2-arylimidazo[2,1-b] [1,3,4]thiadiazol-6-yl)-2H-chromen-2-one against different MDCK cell cultures, HeLa cell cultures, vero cell cultures, CRFK cell cultures is reported. Among the tested compounds, inhibitory effects of compounds (CH-69 to CH-84) on the proliferation of murine leukemia cells (L1210) and human T-lymphocyte cells (CEM) and human cervix carcinoma cells (HeLa).
HeLa • MDCK • CRFK • Thymidine kinase-deficient (TK-) HSV-1 Kos strain • Herpes simplex virus
Levamisole was introduced by Janssen pharmaceutica in 1966 as anthelmintic agent to treat worm infestations in both humans and animals [1]. Later it was withdrawn from the market because of the serious side effects like agranulocytosis [2]. After being pulled out, the molecule has been tested in combination with fluorouracil to treat colon cancer. Evidence from clinical trials supports its addition to fluorouracil therapy to benefit patients with colon cancer [3].
Chemically levamisole is imidazothiazole derivative. Like levamisole, the modified molecule ‘‘Imidazo [2,1-b][1,3,4]thiadiazole’’ also bears anticancer property (Figure 1).
There are two types of bicyclic imidazo [2,1-b]-1,3,4-thiadiazole ring systems are possible. Both the ring systems have nitrogen as a bridgehead atom at 4th position. It is pseudo aromatic in nature containing imidazole as electron rich centre and desired substitution can be done at 2nd, 5th and 6th position by starting with appropriate synthons (Figure 2).
Other researchers have shown imidazo[2,1-b][1,3,4]thiadiazole nucleus as an useful scaffold for the development of novel anticancer agent [4-8].
Based on above discussion, 2-naphthyl-6-aryl-imidazo[2,1-b] [1,3,4]-thiadiazole nucleus has been taken as the target molecule for the thesis entitled “synthesis of levamisole derivatives for anti-cancer activity”.
All chemicals procured for the proposed research work is of high purity. Purity of all chemicals to be confirmed by TLC and solvents to be used after distillation. Proposed research work is comprised of following steps:
1) General method of synthesis of 2-amino-5-substituted-1,3,4- thiadiazole
0.034 M of Phosphorous oxychloride was added drop-wise to mixture of 0.01 M of carboxylic acid [E] and thiosemicarbazide [F] with constant stirring. The reaction mixture was refluxed for one hour, cooled and added to 250 ml of ice-cold water and neutralized with 10% potassium hydroxide solution. The precipitate of 2-amino-5- substituted-1,3,4-thiadiazole [G] was filtered, washed with water and crystallized from DMF-ethanol mixture.
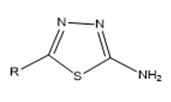
2-amino-5-substituted-1,3,4-thiadiazole
2) General method of synthesis of 3-(2-substituted imidazo[2,1- b]-1,3,4-thiadiazol-6-yl)-2H-chromen-2-one
Equimolar quantity of 3-(2-bromoacetyl)-2H-chromen-2-one [D] and 5-substituted-1,3,4-thiadiazole-2-amine [G] in ethanol was refluxed for 10 hours-12 hours. The reaction mixture was poured in ice-cold water and pH of the solution was adjusted to 7.0 with aqueous solution of Na2CO3 to get 3-(2-substituted imidazo [2,1- b]-1,3,4-thiadiazol-6-yl)-2H-chromen-2-one. The compound so obtained was purified from chloroform-ethanol mixture.
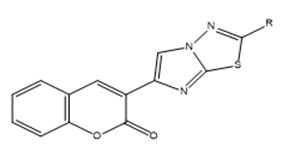
3-(2-substituted imidazo[2,1-b]-1,3,4-thiadiazol-6-yl)-2Hchromen- 2-one
3) General method of synthesis of 5-bromo-3-(2-substituted imidazo[2,1-b]-1,3,4-thiadiazol-6-yl)-2H-chromen-2-one
To a well stirred mixture of 0.0050 M of anhydrous sodium acetate and 0.0025 M of an appropriate 3-(2-substituted imidazo[2,1-b]-1,3,4- thiadiazol-6-yl)-2H-chromen-2-one, 0.0025 M of bromine was added drop wise at room temperature. The stirring was stirred for 1 hour and later poured into ice cold water. The separated solid was filtered and recrystallized from chloroform-ethanol mixture. Physical constant values are given in Table 1.
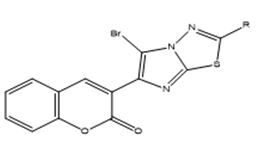
5-bromo-3-(2-substituted imidazo[2,1-b]-1,3,4-thiadiazol-6-yl)-2Hchromen- 2-one:
| Code | R | Nature | % Yield | M.P (°C) | M.F | M.W | Rf Value |
|---|---|---|---|---|---|---|---|
| CH-69 | 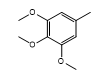 |
Yellow, amorphous | 62 | 210-212 | C22H17N3O5S | 435.45 | 0.56 |
| CH-70 | 2-Methyl thiophene | Brown, crystalline | 40 | 196-98 | C18H11N3O2S2 | 365.43 | 0.54 |
| CH-71 | 0 | Brown, amorphous | 68 | 222-224 | C14H9N3O2S | 283.3 | 0.52 |
| CH-72 | Phenyl | White, amorphous | 65 | 278-80 | C19H11N3O2S | 345.37 | 0.5 |
| CH-73 | Thiophene | Brown, crystalline | 70 | 290-292 | C17H9N3O2S2 | 351.4 | 0.77 |
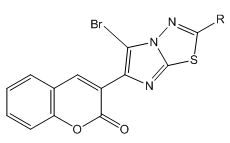
5-bromo-3-(2-substituted imidazo [2,1-b]-1,3,4-thiadiazol-6-yl)-2Hchromen- 2-one
4) General method of synthesis of 5-thiocyanato-3-(2-substituted imidazo[2,1-b]-1,3,4-thiadiazol-6-yl)-2H-chromen-2-one
0.0025 M of bromine in glacial acetic acid (10 ml) was added drop wise at 0°C to a solution of 0.0025 M of 3-(2-substituted imidazo[2,1- b]-1,3,4-thiadiazol-6-yl)-2H-chromen-2-one and 0.004 M of potassium thiocyanate in 10 ml of glacial acetic acid,. The reaction mixture was further stirred for 1 hour at 15°C-18°C, after which it was poured into ice cold water. Solid separated was filtered and recrystallized from the mixture of chloroform/ethanol. Physical constant values are given in Table 2.
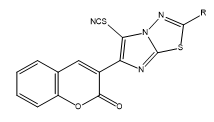
5-thiocyanato-3-(2-substituted imidazo[2,1-b]-1,3,4-thiadiazol-6- yl)-2H-chromen-2-one
| Code | R | Nature | % Yield | M.P (°C) | M.F | M.W | Rf |
|---|---|---|---|---|---|---|---|
| CH-74 |  |
Yellow, amorphous | 55 | 218-220 | C22H16BrN3O5S | 514.35 | 0.63 |
| CH-75 | -CH3 | White, amorphous | 50 | 199-200 | C14H8BrN3O2S | 362.2 | 0.55 |
| CH-76 | Thiophene | Brown, amorphous | 60 | 248-249 | C17H8BrN3O2S2 | 430.29 | 0.48 |
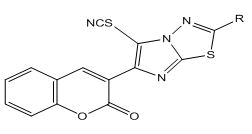
5-thiocyanato-3-(2-substituted imidazo[2,1-b]-1,3,4-thiadiazol-6-
yl)-2H-chromen-2-one
5) General method of synthesis of 5-formyl-3-(2-substituted imidazo[2,1-b]- 1,3,4-thiadiazol-6-yl)-2H-chromen-2-one
0.002 M of 3-(2-substituted imidazo[2,1-b]-1,3,4-thiadiazol-6- yl)-2H-chromen-2-one was added to the freshly prepared Villsmeier- Haack Reagent (prepared by the adding 0.75 ml of POCl3 drop wise to 5 ml of DMF at 0°C-5°C for 30 min) at room temperature. Stirring was continued for 4 hours at 80°C-90°C. The resulting reaction mixture was poured into ice cold water and neutralized to pH 7 with cold aqueous solution of sodium carbonate (Figures 3 and 4). The solid so obtained was filtered and recrystallized from ethanol (Tables 3-6).
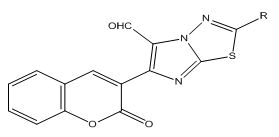
5-formyl-3-(2-substituted imidazo[2,1-b]- 1,3,4-thiadiazol-6-yl)-2Hchromen- 2-one
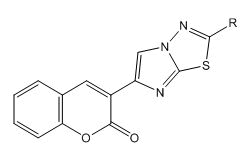
3-(2-substituted imidazo[2,1-b]-1,3,4-thiadiazol-6-yl)-2Hchromen- 2-ones
| Code | R | Nature | % Yield | M.P (°C) | M.F | M.W | Rf |
|---|---|---|---|---|---|---|---|
| CH-77 | 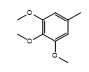 |
Yellow, amorphous | 70 | 242-244 | C23H16N4O5S2 | 492.53 | 0.44 |
| CH-78 | 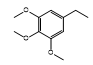 |
White, amorphous | 55 | 180-182 | C24H18N4O5S2 | 506.55 | 0.56 |
![]()
5-formyl-3-(2-substituted imidazo [2,1-b]-1,3,4-thiadiazol-6-yl)-2H-chromen-2-one
| Code | R | Nature | % Yield | M.P (°C) | M.F | M.W | Rf Value |
|---|---|---|---|---|---|---|---|
| CH-79 | 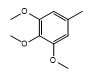 |
Light, yellow | 35 | 192-194 | C23H17N3O6S | 463.46 | 0.54 |
| CH-80 | 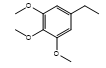 |
White, amorphous | 40 | 178-180 | C24H19N3O6S | 477.49 | 0.66 |
| CH-81 | Phenyl | Brown, amorphous | 45 | 250-252 | C20H11N3O3S | 373.38 | 0.72 |
| CH-82 | Thiophene | Brown, crystalline | 40 | 278-280 | C18H9N3O3S2 | 379.41 | 0.58 |
| Compound code | Spectral peaks (cm-1) | Molecular stretch |
|---|---|---|
| CH-69 | 3050.01, 2972.73-2734 | C-H (aromatic) |
| 1724.05, 1590.99 | C-H (aliphatic) | |
| 1476.24 | >C=O | |
| >C=N | ||
| >C=C | ||
| CH-70 | 3034.44 | C-H (aromatic) |
| 2968.87-2911.99 | -C-H (aliphatic) | |
| 1716.34 | >C=O (Ketone) | |
| 1605.45 | >C=N | |
| 1480.1 | >C=C | |
| CH-71 | 3042.16 | -C-H (aromatic) |
| 2899.45-2844.49 | -C-H (aliphatic) | |
| 1722.12 | >C=O (Ketone) | |
| 1609.31 | >C=N | |
| 1462.74 | >C=C | |
| CH-72 | 3046.98 | -C-H (aromatic) |
| 2972.73 | -C-H (aromatic) | |
| 1718.26 | >C=O | |
| 1606.41 | >C=N | |
| 1432.85 | >C=C | |
| CH-73 | 3058.55 | -C-H (aromatic) |
| 2966.95-2826.17 | -C-H (aliphatic) | |
| 1713.44 | >C=O (Ketone) | |
| 1606.41 | >C=N | |
| 1471.42 | >C=C | |
| CH-74 | 2997.8 | C-H (aromatic) |
| 2942.84-2826.17 | C-H (aliphatic) | |
| 1738.51 | >C=O | |
| 1610.27 | >C=N | |
| 1478.17 | >C=C | |
| CH-75 | 3045.05 | -C-H (aromatic) |
| 2924.52-2961.16 | -C-H (aliphatic) | |
| 1729.83 | >C=O (Ketone) | |
| 1604.48 | >C=N | |
| 1471.42 | >C=C | |
| CH-76 | 3052.76 | -C-H (aromatic) |
| 2942.84-2765.42 | -C-H (aliphatic) | |
| 1729.83 | >C=O (Ketone) | |
| 1597.41 | >C=N | |
| 1471.42 | >C=C | |
| CH-77 | 3028.66 | C-H (aromatic) |
| 2979.48-2833.48 | C-H (aliphatic) | |
| 2167.6 | -CN | |
| 1707.66 | >C=O | |
| 1610.27 | >C=N | |
| 1466.6 | >C=C | |
| CH-78 | 2942.84-2747.10 | -C-H (aliphatic) |
| 2158.92 | -CN | |
| 1702.84 | >C=O | |
| 1604.48 | >C=N | |
| 1465.83 | >C=C | |
| CH-79 | 2942.84-2836.77 | -C-H (aliphatic) |
| 1721.16 | >C=O (Ketone) | |
| 1677.77 | >C=O (Aldehyide) | |
| 1589.06 | >C=N | |
| 1474.31 | >C=C | |
| CH-80 | 3001.2 | -C-H (aromatic) |
| 2906.20-2747.10 | -C-H (aliphatic) | |
| 1716.34 | >C=O (Ketone) | |
| 1654.62 | >C=O (Aldehyde) | |
| 1598.7 | >C=N | |
| 1467.56 | >C=C | |
| CH-82 | 3061.44 | -C-H (aromatic) |
| 2972.73-2869.56 | -C-H (aliphatic) | |
| 1712.48 | >C=O (Ketone) | |
| 1664.27 | >C=O (Aldehyde) | |
| 1610.27 | >C=N | |
| 1475.28 | >C=C |
| Compound code | Chemical shift value (δ) in ppm and proton nature |
|---|---|
| CH-69 | 8.68 (1H, s, ar.), 8.60 (1H, s, ar.), 7.88-7.85 (1H, m, ar.), 7.67-7.57 (1H, m, ar.), 7.45 (1H, d, J=8), 7.37 (1H, t J=16), 7.17 (2H, s, ar.), 3.89 (6H, s, 2-OCH3), 3.75 (3H,s,-OCH3). |
| CH-70 | 8.67 (1H, s, ar.), 8.58 (1H, s, ar.),7.88-7.86 (1H, m, ar.), 7.63-7.59 (1H, m, ar.), 7.52-7.50 (1H, d, ar.), 7.47 (1H, d, J=8), 7.38 (1H, t, j=16.), 7.16-7.15 (1H, m, ar.), 4.73 (2H, s, -CH2). |
| CH-71 | 8.64 (1H, s, ar.), 8.52 (1H, s, ar.), 7.87-7.85 (1H, m, ar.), 7.62-7.58 (1H, m, ar.), 7.46 (1H, d, j=8.), 7.38 (1H, t, j=16), 2.74 (3H, s, -CH3). |
| CH-72 | 8.72 (1H, s, ar.), 8.68 (1H, s, ar.), 7.99 (2H, d, j=8), 7.91 (1H, d, j=8), 7.65-7.59 (4H, m, ar.), 7.49 (1H, d, j=8), 7.40 (1, t, j=16). |
| CH-73 | 10.03 (1H, s, -CHO), 8.57 (1H, s, ar.), 8.01-7.97 (3H, m, ar.), 7.92-7.89 (1H, m, ar.), 7.73-7.69 (2H, m, j=16), 7.52 (1H, d, j=8), 7.43 (1H, t, j=16), 7.32-7.30 (1H, m, ar.) |
| CH-74 | 8.39 (1H, s, ar.), 7.87-7.85 (1H, m, ar.), 7.70-7.65 (1H, m, ar.), 7.49 (1H, d, J=8), 7.41 (1H, t, J=16), 7.21 (2H, s, ar.), 3.92 (6H, s, 2-OCH3), 3.77 (3H, s, -OCH3). |
| CH-75 | 8.33 (1H, s, ar.), 7.85-7.82 (1H, m, ar.), 7.68-7.64 (1H, m, ar.), 7.47 (1H, d, j=8), 7.40 (1H, t, j=16), 2.78 (3H, s, -CH3). |
| CH-76 | 8.70 (1H, s, -ar.), 8.63 (1H, s, ar.), 7.97-7.95 (1H, m, ar.), 7.90-7.88 (2H, m, ar.), 7.64-7.60 (1H, m, ar.), 7.48 (1H, d, j=8), 7.40 (1H, t, j=16), 7.30-7.28 (1H, m, ar.) |
| CH-77 | 8.55 (1H, s, ar.), 7.92-7.90 (1H, m, ar.), 7.73-7.69 (1H, m, ar.), 7.53 (1H, d, J=8), 7.44 (1H, t, J=16), 7.26 (2H, s, ar.), 3.93 (6H, s, 2-OCH3), 3.78 (3H, s, -OCH3). |
| CH-78 | 8.47 (1H, s, ar.), 7.89 (1H, d, j=8.), 7.69 (1H, t, j=16), 7.51 (1H, d, j=8.), 7.42 (1H, t, j=16.), 6.79 (2H, s, ar.), 4.49 (2H, s, -CH2), 3.77 (6H, s, 2-OCH3), 3.65 (3H, s, -OCH3). |
| CH-79 | 10.07 (1H, s, -CHO), 8.59 (1H, s, ar.), 7.92 (1H, d, j=8), 7.71 (1H, t, J=16), 7.52 (1H, d, J=8), 7.43 (1H, t, j=16), 7.25 (2H, s, ar.), 3.93 (6H, s, -OCH3), 3.77 (6H, s, -OCH3). |
| CH-80 | 10.05 (1H, s, -CHO), 8.52 (1H, s, ar.), 7.90 (1H, d, j=8), 7.70 (1H, t, j=16), 7.50 (1H, d, j=8), 7.42 (1H, t, j=16), 6.78 (2H, s, ar.), 4.47 (2H, s, -CH2), 3.77 (6H, s, 2-OCH3), 3.65 (3H, s, -OCH3). |
| CH-82 | 8.37 (1H, s, -ar.), 8.00-7.99 (1H, m, ar.), 7.94-7.93 (1H, m, ar.), 7.87-7.84 (1H, m, ar.), 7.69-7.65 (1H, m, ar.), 7.48 (1H, d, j=8), 7.43-7.38 (1H, m, ar.), 7.31-7.29 (1H, m, ar.) |
2-aralkyl-6-aryl-imidazo-[2,1-b][1,3,4]-thiadiazoles
Series of 2,6-disubstituted-imidazothiadiazoles were prepared. The FTIR spectra find peaks in the range of 3125-3008 and 2969 cm-1-2764 cm-1 for aromatic and aliphatic -CH respectively. The imine (-C=N) and -C=C (Ar.) stretching observed between 1621 cm-1-1563 cm-1 and 1545 cm-1-1463 cm-1 respectively. Presence of -C=O stretching at 1702 cm-1 and 1716 cm-1 respectively. The 1H-NMR spectra showed peaks between 8.92-8.49, 8.25-6.93, and 4.95 -4.35 δδ ppm for imidazole -CH, aromatic -CH, and -CH2 protons respectively. The 2H-chromen-2-one proton of CH-8 and CH-15 appeared at 8.65 δ and 8.55 δ ppm respectively. The -OCH3 protons appeared between 3.75 δ -3.74 δ ppm for CH-2, 8 and CH-13. The - CH3 protons appeared at 2.29 δ ppm in CH-14. 13C-NMR spectra of CH-2 and CH-8 had shown peaks between 165-110 and 37 δ -36 δ ppm for aromatic and -CH2 carbons respectively. The methyl carbons (-OCH3) of CH-2 and 8 appeared at 55 δ ppm. The mass spectra of CH-2 and CH-8 had shown molecular ion peaks in positive mode at m/z 340.02 and 390.08 respectively. The FTIR, 1HNMR, 13C-NMR and HRMS data were summarized.
Anticancer activity in human and murine tumor cell lines
All the compounds (CH-69 to CH-84) were evaluated for their cytostatic activity against human HeLa cervix carcinoma cells, human CEM CD4þ T-lymphocytes as well as murine L1210 cells. All assays were performed in 96-well microtiter plates. To each well were added (5-7.5) × 104 tumor cells and a given amount of the test compound.
The cells were allowed to proliferate for 48 h (murine leukemia L1210 cells) or 72 h (human lymphocytic CEM and human cervix carcinoma HeLa cells) at 37°C in a humidified CO2 controlled atmosphere. At the end of the incubation period, the cells were counted in a coulter counter. The IC50 (50% inhibitory concentration) was defined as the concentration of the compound that inhibited cell proliferation by 50%.
Antiviral activity assays
The compounds were evaluated against the following viruses: herpes simplex virus type 1 (HSV-1) strain Kos, Thymidine Kinase- Deficient (TK-) HSV-1 Kos strain resistant to ACV (ACVr), Herpes Simplex Virus Type 2 (HSV-2) strains Lyons and G, Varicella Zoster Virus (VZV) strain Oka, TKVZV strain 07-1, Human Cytomegalovirus (HCMV) strains AD-169 and Davis, a clinical isolate of Adenovirus type 2 (Ad2), Human Herpes Virus 6 subtype A (HHV-6A) strain GS, vaccinia virus Lederle strain, Respiratory Syncitial Virus (RSV) strain Long, Vesicular Stomatitis Virus (VSV), Coxsackie B4, parainfluenza 3, reovirus-1, Sindbis, Punta Toro, Yellow Fever Virus (YFV), human immunodeficiency virus type 1 strain IIIB, human immunodeficiency virus type 2 strain ROD, and Hepatitis C Virus (HCV). The antiviral, other than anti-HIV and anti-HCV, assays were based on inhibition of virus-induced cytopathicity or plaque formation in Human Embryonic Lung (HEL) fibroblasts, African green monkey cells (Vero), Human Epithelial cells (HeLa), or Human T-lymphoblasts (HSB-2), according to previously established procedures. Confluent cell cultures in microtiter 96-well plates were inoculated with 100 CCID50 of virus (1 CCID50 being the virus dose to infect 50% of the cell cultures) or with 20 Plaque-Forming Units (PFUs). After a 1 h-2 h adsorption period, residual virus was removed, and the cell cultures were incubated in the presence of varying concentrations of the test compounds. Viral cytopathicity or plaque formation (VZV) was recorded as soon as it reached completion in the control virus-infected cell cultures that were not treated with the test compounds. Antiviral activity was expressed as the EC50, or the concentration required to reduce virus induced cytopathogenicity or viral plaque formation by 50%.
Cytotoxicity assays
Cytotoxicity measurements were based on the inhibition of cell growth. HEL cells were seeded at a rate of 5 cells-103 cells/well into 96-well microtiter plates and allowed to proliferate for 24 h. Then, medium containing different concentrations of the test compounds was added. After 3 days of incubationat 37°C, the cell number was determined with a coulter counter. The cytostatic concentration was calculated as the CC50, or the compound concentration required to reduce cell proliferation by 50% relative to the number of cells in the untreated controls. CC50 values were estimated from graphic plots of the number of cells (percentage of control) as a function of the concentration of the test compounds. Alternatively, cytotoxicity for cell morphology was expressed as the Minimum Cytotoxic Concentration (MCC), or the compound concentration that caused a microscopically detectable alteration of cell morphology.
Inhibitory effects of compounds (CH-69 to CH-84) on the proliferation of murine leukemia cells (L1210) and human Tlymphocyte cells (CEM) and human cervix carcinoma cells (HeLa) (Tables 7 and 8).
| Compound | IC50* (µM) | ||
|---|---|---|---|
| L1210 | CEM | HeLa | |
| CH-69 | >250 | >250 | >250 |
| CH-70 | 211 ± 14 | 138 ± 35 | >250 |
| CH-71 | ≥ 250 | 196 ± 4 | >250 |
| CH-72 | >250 | >250 | >250 |
| CH-73 | >250 | >250 | >250 |
| CH-74 | >250 | >250 | >250 |
| CH-75 | >250 | >250 | >250 |
| CH-76 | >250 | >250 | >250 |
| CH-77 | ≥ 250 | 165 ± 6 | >250 |
| CH-78 | NT | NT | NT |
| CH-79 | >250 | >250 | >250 |
| CH-80 | NT | NT | NT |
| CH-81 | NT | NT | NT |
| CH-82 | >250 | >250 | >250 |
| CH-83 | 23 ± 1 | 3.5 ± 0.8 | 9.5 ± 0.4 |
| CH-84 | 1.6 ± 0.4 | 0.77 ± 0.06 | 0.38 ± 0.03 |
*50% inhibitory concentration.
| Cytotoxicity | Antiviral EC50C | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound | CC50a | Minimum cytotoxic concentrationb | Influenza A/H1N1 A/Ned/378/05 | Influenza A/H3N2 A/HK/7/87 | Influenza B B/Ned/537/05 | |||
| Visual CPE score | MTS | Visual CPE score | MTS | Visual CPE score | MTS | |||
| CH-69 | 52.8 | 100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-70 | >100 | ≥ 100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-71 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-72 | >100 | ≥ 100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-73 | >100 | ≥ 100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-74 | >100 | ≥ 20 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-75 | 51.4 | ≥ 20 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-76 | >100 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-77 | >100 | 20 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-78 | NT | NT | NT | NT | NT | NT | NT | NT |
| CH-79 | >100 | >100 | 50 | 32.8 | 100 | >100 | 20 | 11.7 |
| CH-80 | NT | NT | NT | NT | NT | NT | NT | NT |
| CH-81 | NT | NT | NT | NT | NT | NT | NT | NT |
| CH-82 | >100 | ≥ 100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-83 | >100 | ≥ 20 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-84 | 2 | ≥ 0.8 | >100 | >100 | >100 | >100 | >100 | >100 |
| Zanamivir | >100 | >100 | 0.3 | 0.05 | 4 | 11.6 | 0.09 | 0.07 |
| Ribavirin | >100 | >100 | 20 | 4 | 20 | 9.1 | 6.8 | 3.1 |
| Amantadine | >200 | >200 | 20 | 9.2 | 4 | 2.8 | >200 | >200 |
| Rimantadine | >200 | >200 | 40 | 15.9 | 0.8 | 0.1 | >200 | >200 |
Note: a50% Cytotoxic concentration, as determined by measuring the cell viability with the colorimetric formazan-based MTS assay.
bMinimum compound concentration that causes a microscopically detectable alteration of normal cell morphology.
c50% Effective concentration, or concentration producing 50% inhibition of virus-induced cytopathic effect, as determined by visual scoring of the CPE, or by measuring the cell viability with the colorimetric formazan-based MTS assay.
MDCK cells: Madin Darby canine kidney cells
Data indicating antiviral activity are shown in red font, and marked in yellow if the SI (ratio of MCC to EC50) is five or higher (Tables 9-12).
Note that the SI can not be accurately calculated for compounds showing no cytotoxicity at the highest concentration tested (100 µM).
| EC50b | |||||||
|---|---|---|---|---|---|---|---|
| Compound | Minimum cytotoxic concentrationa | Herpes simplex virus-1 (KOS) | Herpes simplex virus-2 (G) | Herpes simplex virus-1 TK-KOS ACVr | Vaccinia virus | Adeno virus-2 | Human Coronavirus (229E) |
| CH-69 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-70 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-71 | 100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-72 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-73 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-74 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-75 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-76 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-77 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-78 | NT | NT | NT | NT | NT | NT | NT |
| CH-79 | 20 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-80 | NT | NT | NT | NT | NT | NT | NT |
| CH-81 | NT | NT | NT | NT | NT | NT | NT |
| CH-82 | 100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-83 | 100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-84 | 20 | >100 | >100 | >100 | >100 | >100 | >100 |
| Brivudin | >250 | 0.08 | 112 | >250 | 10 | - | - |
| Cidofovir | >250 | 5 | 2 | 2 | 14 | 10 | - |
| Acyclovir | >250 | 0.08 | 0.8 | >250 | >250 | - | - |
| Ganciclovir | >100 | 0.3 | 0.094 | 20 | >100 | - | - |
| Zalcitabine | >250 | - | - | - | - | 5.8 | - |
| Alovudine | >250 | - | - | - | - | 10 | - |
| UDA | >100 | - | - | - | - | - | 1.8 |
| Ribavirin | >250 | - | - | - | - | - | 85 |
Note: aRequired to cause a microscopically detectable alteration of normal cell morphology; bRequired to reduce virus-induced cytopathogenicity by 50%.
| EC50b | |||||
|---|---|---|---|---|---|
| Compound | Concentration unit | Minimum cytotoxic concentrationa | Vesicular stomatitis virus | Coxsackie virus B4 | Respiratory syncytial virus |
| 69 | µM | >100 | >100 | >100 | >100 |
| 70 | µM | >100 | >100 | >100 | >100 |
| 71 | µM | >100 | >100 | >100 | >100 |
| 72 | µM | >100 | >100 | >100 | >100 |
| 73 | µM | >100 | >100 | >100 | >100 |
| 74 | µM | 100 | >100 | >100 | >100 |
| 75 | µM | >100 | >100 | >100 | >100 |
| 76 | µM | >100 | >100 | >100 | >100 |
| 77 | µM | 100 | >100 | >100 | >100 |
| 78 | -- | NT | NT | NT | NT |
| 79 | µM | ≥100 | >100 | >100 | >100 |
| 80 | -- | NT | NT | NT | NT |
| 81 | -- | NT | NT | NT | NT |
| 82 | µM | >100 | >100 | >100 | >100 |
| 83 | µM | 100 | >100 | >100 | >100 |
| 84 | µM | 4 | >100 | >100 | >100 |
| DS-10.000 | µg/ml | >100 | 1.4 | >100 | 0.5 |
| Ribavirin | µM | >250 | 5 | 146 | 3.4 |
Note: aRequired to cause a microscopically detectable alteration of normal cell morphology; bRequired to reduce virus-induced cytopathogenicity by 50%.
| EC50b | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | Concentration unit | Minimum cytotoxic concentrationa | Para-influenza-3 virus | Reovirus-1 | Sindbis virus | Coxsackie virus B4 | Punta virus Toro | Yellow Fever virus |
| CH-69 | µM | ≥ 20 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-70 | µM | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-71 | µM | 100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-72 | µM | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-73 | µM | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-74 | µM | ≥20 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-75 | µM | 100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-76 | µM | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-77 | µM | ≥20 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-78 | -- | NT | NT | NT | NT | NT | NT | NT |
| CH-79 | µM | 100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-80 | -- | NT | NT | NT | NT | NT | NT | NT |
| CH-81 | -- | NT | NT | NT | NT | NT | NT | NT |
| CH-82 | µM | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-83 | µM | 100 | >100 | >100 | >100 | >100 | >100 | >100 |
| CH-84 | µM | ≥ 0.8 | >100 | >100 | >100 | >100 | >100 | >100 |
| DS-10.000 | µg/ml | >100 | >100 | >100 | >100 | 58 | 50 | 0.4 |
| Ribavirin | µM | ≥ 250 | 19 | 111 | >250 | >250 | 25 | >250 |
| Mycophenolic acid | µM | >100 | 0.4 | 0.6 | >100 | >250 | 2.3 | 0.8 |
Note: aRequired to cause a microscopically detectable alteration of normal cell morphology; bRequired to reduce virus-induced cytopathogenicity by 50%.
| EC50b | ||||
|---|---|---|---|---|
| Compound | Concentration unit | CC50a | Feline Corona Virus (FIPV) | Feline Herpes Virus |
| CH-69 | µM | >100 | >100 | >100 |
| CH-70 | µM | >100 | >100 | >100 |
| CH-71 | µM | >100 | >100 | >100 |
| CH-72 | µM | >100 | >100 | >100 |
| CH-73 | µM | >100 | >100 | >100 |
| CH-74 | µM | >100 | >100 | >100 |
| CH-75 | µM | >100 | >100 | >100 |
| CH-76 | µM | >100 | >100 | >100 |
| CH-77 | µM | 13 | >100 | >100 |
| CH-78 | -- | NT | NT | NT |
| CH-79 | µM | 39.6 | >100 | >100 |
| CH-80 | -- | NT | NT | NT |
| CH-81 | -- | NT | NT | NT |
| CH-82 | µM | >100 | >100 | >100 |
| CH-83 | µM | >100 | >100 | >100 |
| CH-84 | µM | 4.9 | >100 | >100 |
| HHA | µg/ml | >100 | 3.3 | 2.7 |
| UDA | µg/ml | >100 | 14.4 | 9.1 |
| Ganciclovir | µM | >100 | >100 | 1.6 |
Note: a50% Cytotoxic concentration, as determined by measuring the cell viability with the colorimetric formazan-based MTS assay; b50% Effective concentration, or concentration producing 50% inhibition of virus-induced cytopathic effect, as determined by measuring the cell viability with the colorimetric formazan-based MTS assay.
CRFK cells: Crandell-Rees Feline Kidney cells.
Chemical Sciences Journal received 912 citations as per Google Scholar report