Research Article - (2020) Volume 11, Issue 1
Received: 28-Jan-2020
Published:
21-Feb-2020
, DOI: 10.37421/jch.2020.11.552
Citation: Mohammad Y Alfaifi, Tamer MM Abuamara, Mohamed E Amer, Mohamed SM Nasr, Wagih M Abd-Elhay, Laila E EI-Moselhy, et al. Use of Secondary Metabolites Derived from Aspergillus Species as Anticancer Agents and Related Histological and Genetic Alterations: In Vitro Study. J Cytol Histol 10 (2020) doi: 10.37421/jch.2020.11.552
Copyright: © 2020 Alfaifi MY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
The present work aimed to use natural derived fungal secondary metabolites as anticancer agents concerning cytotoxicity, apoptotic, genetic and histopathological profile. It was noticed that Asp. Terrius (Asp. T), Flavous (Asp. Fl) and Fumegatgus (Asp. Fu) induced variable toxic potential that was cell type, secondary metabolite type and concentration dependent. CaCo-2 cells showed less sensitivity than HuH-7 and in turn the IC50 was variable. Also, the apoptotic potential of Asp. species derived fungal secondary metabolites was proven via detection of up regulated pro apoptotic genes and down regulation of anti-apoptotic genes. The expression rate was cell type dependent. Concurrently apoptotic profile was accompanied with cellular DNA accumulation at the G2/M phase, an insignificant accumulation during the G0/G1 phase but there was not during the Pre-G1 and S phases. Also, there were a characteristic apoptotic features of treated cells presented as abnormal intra-nuclear eosinophyllic structures, necrotic cells with mixed euchromatin and heterochromatin, ruptured cell membranes, intranuclear eosinophyllic structures, apoptotic cells with irregular cellular and nuclear membranes, peripheral chromatin condensation and necrotic swollen cells with mixed euchromatin and heterochromatin. It can be concluded that A. secondary metabolites are promising agents can be used as a supplementary agents to current cancer drugs regimen applied.
Aspergillus spp • Cytotoxicity • Apoptosis • Histopathology • Flowcytometry
Natural products driven molecules comprise approximately 50% of the drugs presently used for clinical purposes. Regarding anticancer drugs, 63% of these derivatives fall into this category [1-3]. Despite the high number of available drugs, there is a growing need to develop more specific affordable agents to manage cancers, particularly chemo-resistant tumors [4]. Consequently, the need for diversity of molecules for their pharmacological activity led to a larger growth of natural anticancer drug production. The potential of marine natural products has drew attention of several researchers for the wide biodiversity in the marine environment and related secondary metabolites and their pharmacological potential specially those exhibited anticancer potential [5,6]. As a significant portion of the bioactive metabolites, thought originally to be have products often synthesized by symbiotic microbiota [7]. Researchers have paid more attention to microorganisms as a renewable source of bioactive natural products. More specifically, fungi from the marine environment those have shown a great potential as suggested by the diversity of secondary metabolites [8]. The largest number of total compounds in the literature have the overall highest number of novel metabolites of bioactivity specially against cancer and microbes. So, many researchers in the last 10 years drew attention for isolation and antitumor activity evaluation of the secondary metabolites from marine sponges, soil fungi and marine fungi (E. cristatum) as well, as it was proved that sponge associated fungi were used to evaluate their inhibitory effect of different cancer cell lines specially the ethyl acetate extract of Eurotium cristatum (ECE) in breast adenocarcinoma (MCF-7), non-small lung cancer (NCI-H460), and melanoma (A375-C5) cell lines. In addition, 2-(2‟,3-epoxy-1‟,3‟-heptadienyl)-6-hydroxy-5-(3-methyl-2- butenyl) benzaldehyde physcion (1,8-dihydroxy-6-methoxy-3-methyl-9,10- anthracenedione), and echinulin secondary metabolites were isolated from the ECE and growth inhibitory potential of the same human tumor cell lines was tested. Some members of the genus Eurotium have been previously investigated for their secondary metabolites anticancer potential [9-12]. So, the present study aimed to in vitro evaluate the extracted secondary metabolites of Asp. species (T, Fl, Fu) isolated from the Egyptian water resources as anticancer derivatives concerning liver (HuH-7) and colon cancer (Caco-2) cancer cell lines and to evaluate the toxicological, pathological effects and related changes induced. Also, evaluation of cell cycle and apoptotic profile and related apoptotic genes was carried out.
Fungal isolation and growth conditions
Fungal isolates were cultured on malt extract agar medium (malt extract, 20 g; peptone, 1 g; dextrose, 20 g; agar, 20 g; and distilled water, 1 L) was used for isolation, cultivation, and identification of the fungal isolates and test organisms. Nutrient Agar (Peptone, 5 g; beef extract, 3g; sodium chloride, 3 g, Agar, 20 g; and distilled water, 1 L) was used for cultivation of bacterial test organisms. For production of secondary metabolites, yeast extract sucrose agar (yeast extract, 20 g; sucrose, 150 g; agar, 20 g; and distilled water, 1L) was used. The fungal isolate was identified in the Environmental monitoring center, the Holding company for production of vaccines, Sera and Drugs (VACSERA), Cairo, Egypt according to the center protocols.
Fungal strains
Fugal strains were identified using the routine protocols. Asp. T, Asp. Fl and Asp. Fu were cultured on specific fungal growth medium for 21 days. Secondary metabolites were cold centrifuged and sterile filtered using sterile Stericup set (Millipore-USA) under laminar Air flow (Unair-USA). Filtrates were collected using vacuum pump (Millipore –USA). Derived metabolite fluid was tested for sterility using bacterial and fungal media for 7 and 14v days at 37°C and 22°C respectively. Sterile filtrates were liquated as 2 ml/ sterile vials (Griener Bio One - Swiss) and preserved at -80°C till use.
Cell culture/MTT stain
HuH-7 and Caco-2 cells were kindly supplied from the International Center for training and Advanced Researches (ICTAR-Egypt). Cells were maintained according to the instructions of manufacturer and were maintained in RPMI-1640 (Hy-Clone Laboratories, South Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; GIBCO-USA), 100 U/ml penicillin and 100 mg/l streptomycin (Sigma-Aldrich -USA). The cells were cultured in a humidified incubator containing 5% CO at 37°C. Both adherent cell lines were dissociated according to the established protocols, and cell No was adjusted as 2x105/ml in 10% RPMI-1640 growth medium. Cells were dispensed as 100 μL in 96 well plates (TPP-Swiss) plates were incubated at 37°C till cell confluence. The fungal secondary metabolites 500 µg/ml were dispensed as 0.1 ml/well, and 2 fold serially diluted in maintenance medium replacing the exhausted growth medium. Twenty four hrs later the detached cells were washed out using sterile phosphate buffer saline (LONZA-Swiss), 25 μl 5 mg/ml MTT solution in PBS was added to each well for 4 h. Developed Formazan crystals were dissolved using 50 µL DMSO (Sigma-Aldrich, USA) and incubated for 30 minutes at 37°C with periodical shaking. The absorbance of developed color was determined using Elx-800 BioTek micro plate reader at 570 nm wave length According to the optical densities, the viability% was determined according to the following equation
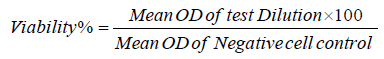
Viability% was plotted against the concentration of secondary metabolites
Evaluation of cytotoxicity of derived metabolites was carried on both liver and colon cancer cell line. Data recorded revealed that the viability percentage was concentration, cell type and Asp. spp dependent as viability increased relatively to the lower concentration. The cytotoxic effect of test metabolites was accompanied with cell rounding, detachment and membrane blipping as shown in Figures 1 and 2 colon cancer cell lines, whereas the IC50 value of Asp. Fv treated HuH-7 and CaCo-2 showed an insignificant difference (P>0.05), while Asp. T showed significant decreased IC50 value in case of CaCo-2 cell line treatment than in case of Caco-2/Asp. Fu (P<0.05), also, Asp. Fu showed insignificant different IC50 value in case of HuH-7 and CaCo-2 (P>0.05) and Asp. Fu showed a significantly reduced IC50 values in both HuH-7 and CaC0-2 (P<0.05) compared with its values in case of Asp. T treated both CaCo-2 and HuH-7cells treated as shown in Figure 3. Apoptosis of colon and liver cancer was monitored via evaluation of pro and anti-apoptotic genes performed. Data recorded revealed that pro apoptotic genes (Bax and P53) were significantly up regulated compared with that of untreated cell control (P<0.05). It was clear that Caco-2 was more sensitive to Asp. Metabolites than in case of HuH-7 but not in case of using Asp. T. In the mean anti-apoptotic gene (BCL-2) was significantly down regulated (P<0.05) in case of cell treatment with Asp.T/HuH-7 and Asp.Fu/Caco-2 respectively and there was an insignificant difference in the rest of cell treated Asp sp metabolites (P>0.05) compared with control. In the meantime there was a significant up regulation of cytochrome - C gene post treatment with i and Asp. Fv/Caco-2/HuH-7 compared with its value in control and rest of treated groups as shown in Figure 4. The cell cycle profile was traced and it was clear that there was a significant (P<0.05) accumulation of treated cell DNA mainly at the G2/M and Pre/G1 phases phases, the highest arrest vales detected in case of cell treatment with Asp. Fu/Caco-2, Asp. FU/HuH-7 compared with rest of treated groups values (P<0.05). Also, there was an insignificant DNA accumulation detected in the G0/G1 and S phase as shown in Figure 5. The IC50 treated cells showed histopathological alteration hyperchromatic nuclei and nuclear pleomorphism. Asp. T metabolite HuH-7 treated cells showed swollen necrotic cells with mixed euchromatin and heterochromatin and ruptured cell membranes. Some cells showed apoptotic features of shrunken cells, nuclei and peripheral condensation of chromatin. Also, some features of secondary necrosis could be detected. Abnormal intranuclear eosinophyllic structures were also observed. In the meantime HuH-7 Asp. Fu metabolites treated cells showed necrotic cells with mixed euchromatin and heterochromatin, ruptured cell membranes, intranuclear eosinophyllic structures, shrunken apoptotic cells with irregular cell and nuclear membranes. Also, Caco-2 AT and Asp.Fu metabolites treated cells showed shrunken apoptotic cells with peripheral condensation of chromatin and necrotic swollen cells with mixed euchromatin and heterochromatin and ruptured cell membrane and necrotic cells with mixed euchromatin and heterochromatin, ruptured cell membranes, intra-nuclear eosinophyllic structures, shrunken apoptotic cells with irregular cell and nuclear membranes.
Regarding the biochemical anticancer potential marker (ROS) of test secondary metabolites, it was noticed that there was a significant elevated ROS in Caco-2 and HuH-7 treated cell lines compared with its level in untreated cell control (P<0.05). On the contrary, there was an insignificant elevated ROS (P>.05), when its level was compared in both Caco-2 and HuH-7n cells treated with the secondary metabolites as shown in Figures 6-9.
Secondary metabolites production from fungi is complex process producing compounds with obscure or unknown functions in the producing organisms. However, many of these metabolites have tremendous importance to humankind, as they display a broad range of useful antibiotic and pharmaceutical activities) Aly. F Mohamed, 2019) unpublished data. So the present study aimed for characterization of Asp. T, Asp.Fl and Asp. Fu derived secondary metabolites anticancer potentials against human liver (HuH-7) and colon cancer cell lines (Caco-2). It was noticed that crowd metabolites exhibited a concentration, secondary metabolites and cell type dependent viability% and IC50 values. And cell cycle phases distribution analysis indicated that the DNA accumulation was detected significantly in the G2-M phase which represents apoptotic dead cells. And a lesser significant arrest was detected at the Go-G1 and S phases. Cell death/ toxicity may be attributed to elevated ROS and depleted GSH concentration, Also, it may be due to the existence of different toxic proteins like Terrein, Terrecyclic acid A and Gliotoxin proteins as recorded (Figure 10) [13,14].
Anticancer activity of secondary metabolites may also, be attributed to different derivatives like Gliotoxin; a dipeptide characterized by the presence of a disulfide bridge across the piperazine ring [15]. The disulfide bridge allows the cross linking with proteins via cysteine residues and generate deleterious reactive oxygen species (ROS) through the redox cycling between the reduced and oxidized forms. This mechanism of ROS generation was believed to be responsible for the toxicity of Gliotoxin [16,17]. Pardo Julian et al. demonstrated that Gliotoxin directly activated the proapoptotic Bcl-2 family member Bak, a constitutive mitochondrial protein in MEF cells [18]. The generated ROS was reported to facilitate cytochrome C release and apoptosis inducing factors from mitochondria, leading to caspase activation, as well as other events that mediate cell death [18]. Also, Terrein can act as a bio-derived anticancer agent as it is a multi-potential protein isolated from endophytic fungus JAS-2, from well recognized medicinal herb Achyranthus aspera. This compound had also exhibited anticancer activity against human lung cancer cell line A-549. Also, Terrein was isolated from various strains of fungi such as Microspora Pencillium Phoma and reported to have anticancer activity against different cell lines [19,20]. Recently Terrein reported to exhibit anti-oxidant activity as it has scavenged 50% DPPH at 112 μgml−1 [21]. The antioxidant property can be correlated with earlier reports as melanogenesis inhibitor [22,23]. It was also reported that Terrein inhibits age-related inflammation by promoting an antioxidant response in aged human diploid fibroblast (HDF) cells [24]. Terrein has been tested against different types of cancer cell lines such as pancreatic cancer, breast cancer, human cervical, skin, and prostate cancer [25,26]. Also, it was reported that filamentous Asp. produces numerous bioactives derivatives such as mycotoxins act as anticancer agents. Despite these new compounds revealing remarkable new bioactivities are still being discovered, including well-known metabolites such as Griseofulvin [1,3-5]. Anticancer natural products primarily produced by Asp., namely polyketides are considered a source of anticancer derivatives and those are belonging to the statin family; cholesterol synthesis inhibitors. The statin structure is based on a dicyclohexene ring system connected to a side chain with a closed lactone ring or an open acid form [25]. Terrein as Polyketide produced by A. terries has been found to inhibit breast cancer by induction of apoptosis with an IC50 value of 1.1 nM in MCF-7 cell line, that made Terrein 100-fold more potent than Taxol against this cell line. Additionally Terrein was found active against pancreatic and liver cancer cell lines PANC-1 (IC50 9.8 μM) and HepG2 (IC50 66.8 μM) [18,25]. Also, Asperlin as small Polyketide was isolated from A. nidulans found to reduces cell proliferation and induce G2/M cell cycle arrest in the human cervical carcinoma HeLa cell line, which was in agreement with the present work recorded data [27,28].
Filamentous fungi are known to produce Griseofulvin (GF) (Figure 11) as reported before with anticancer potential polyketides with different spiroring structures could induce cell proliferation and mitosis in the human cervical cancer cell line (HeLa) with an IC50 value of 20 μM, as well as inhibiting centrosomal clustering in human squamous cancer SCC-114 cell line with an IC50 value of 35 μM [23,24]. The synthetic analog GF-15 increased the inhibitory effect of centrosomal clustering in SCC-114 cells 25-fold with an IC50 value of 0.9 μM [29]. It was further shown that in vivo combined treatment of COLO 205 infected mice with GF-15 and nocodazole as anticancer drug, the anticancer potential of the later was improved followed by a higher% of arrested tumor cells and cellular proliferation inhibition [27]. A. parasiticus as polyketides derived Sequoiamonascin A and B with spiro ring structures showed to have selective anticancer activity against different leukemia and two melanoma cell lines with a median growth inhibitory (GI50) log10 value of -6.00 [27]. Furthermore, sequoiamonascin A showed cytotoxic activity against breast cancer MCF-7, lung cancer NCI-H460 and central nervous system (CNS) cancer SF-268 cell lines, where cell growth can be reduced to 1%–2% when treated with 10 μM sequoiamonascin A, [30]. Concurrently A. parasiticus and A. nidulans proved to produce fungal metabolites like (NoA)Norsolorinic acidtype a and b with a tricyclic structure that could induce cell cycle arrest at the G0/G1 phase of the cell cycle and consequently induced apoptosis in human bladder cancer T-24 and human breast cancer MCF-7 with IC50 values of 10.5 and 12.7 μM, respectively [31,32]. The activity secondary metabolites namely Norsolorinic acid (a) and (b) and Austocystin D derived from Aspregillus species suggests to have anticancer potential [31-34]. Also, Asp. t derived (TC-A)Terrecyclic acid A exhibited cytotoxic activity against human lung cancer NCI-H460, human breast cancer MCF-7, and human CNS cancer SF-268 cell lines with IC50 values of 10.6, 24.1 and 14.7 μM, respectively, and against leukemia in mice P-388 with LD50 values of 63–125 mg/kg [35]. Asp. ustus was later ascribed to the three different species: Asp. calidoustus, Asp. insuetus, and Asp. keveii from the Asp. section Usti [36]. Also, (Ob-A) Ophiobolin A exhibited an inhibitory activity against cancer cell lines includes lung cancer A-549, colon cancer HT-29, melanoma Mel-20, leukemia P-388, and ovarian cancer OVCAR-3 with IC50 values of 0.1, 0.1, 0.1, 0.06 and 0.3 μM, respectively [37,38]. Novel discovered ophiobolin O found to inhibits breast cancer MCF-7 and leukemia P-388 cell lines with IC50 values of 17.9 and 4.7 μM, respectively [39,40]. Regarding the apoptotic profile of Asp. Fl, Asp.T and Asp. Fu, it was reported that the cytotoxicity of test metabolites may be attributed to Terrein and Geliotoxin and other proteins produced by A. species those could induce biochemical changes indicated by depleted GSH and elevated ROS inducing up-regulation of proapoptotic gene and down regulation of anti-apoptotic one in treated liver and colon cancer cell line, these data was approved our reorded data and [18] as well despite their use of murine models recording that Asp. Fu gliotoxin (GT), as a secondary metabolite, is cytotoxic for mammalian cells, but the molecular basis and biological relevance of this toxicity remain speculative. And they showed that GT could induce apoptotic cell death by activating the proapoptotic Bcl-2 family member Bak, but not Bax, to elicit the generation of reactive oxygen species, the mitochondrial release of apoptogenic factors, and caspase-3 activation. Activation of Bak by GT is direct, as it triggers in vitro in a dose-dependent manner the release of cytochrome c from purified mitochondria isolated from wild-type and Bax- but not Bak-deficient cells. Also, reported that resistance to Asp. Fu of mice lacking Bak compared to wild-type mice demonstrates the in vivo relevance of this GT-induced apoptotic pathway involving Bak and suggests a correlation between GT production and virulence. Also, it was reported that Asp. Fu secondary metabolites affect cell toxicity via change in the physiological profile of A-549 as Asp .fu produced a large number of secondary metabolites, contain five main compounds, tryptoquivaline F, fumiquinazoline C, questin, monomethylsulochrin and trypacidin. Those were toxic to the human A-549 lung cell line. Trypacidin was the most toxic, decreasing cell viability and triggering cell lysis, both effects occurring at an IC50 close to 7 mM., Also, trypacidin as secondary metabolite could initiate the intracellular formation of nitric oxide (NO) and hydrogen peroxide (H2O2). This oxidative stress triggers necrotic cell death. The apoptosis pathway, moreover, was not involved in the cell death process as trypacidin did not induce apoptotic bodies or a decrease in mitochondrial membrane potential (MMP). Also, it was reported that extracellular polyscharide derived from Asp. aculeatus designated as WPA and WPB of 28.1 kDa and 21.0 kDa, respectively [41]. WPA composed of mannose and galactose in a molar ratio of 3.9:1.0, while WPB mainly contained mannose. Meanwhile, WPA displayed in vitro a stronger anti-proliferative effect than WPB on HeLa, MCF-7 and MGC- 803 cells. And WPA and WPB could arrest HeLa cells in G2/M phase and induce HeLa cells apoptosis. So, this polysaccharide provides evidence of anticancer potential for treating cervical carcinoma. Unfortunately seldom references concerned in vitro pathological alteration but in vivo. While we tried to explore the related pathological changes induced in HuH-7 and Caco-2 cell lines under the effect of Asp. species derived secondary metabolites. Where, untreated cell control showed a regular tumor cells, cellular pleomorphism and nuclear pleomorphism. Oppositely, treated cells showed apoptotic bodies, irregular cell membranes. Secondary metabolite treated cells showed membrane of neoplastic and necrotic cells with euchromatin, heterochromatin and ruptured cell membrane. Cells also, showed numerous apoptotic and necrotic bodies and nuclear fragmentation post HuH-7 and Caco-2 treatment with secondary metabolites and irregular cell membrane of neoplastic cells.
Finally it can be concluded that secondary metabolites are a promising option to enhance the current anticancer drug therapeutic approaches with a higher safety measure than the drawback of synthetic drugs in addition its affordability.
Conceptualization, A.F.M, T.M.M.A, M.Y.F; Methodology, A.F.M, S.I.B, H.A.A., T.M.M.A., M.E.A., M.S.M.N., W.M.A., L.E.M., T.B.G.; Software, S.I.B., H.A.A., A.F.M.; formal analysis, A.F.M., S.I.B.; data curation, A.F.M., T.M.M.A., M.E.A., L.E.M., M.S.M.N., W.M.A.,T.B.G.; writing,-original draft preparation, A.F.M.; writing-review and editing A.F.M., T.M.M.A, M.E.A;, supervision, A.F.M.; project administration, M.Y.F., S.I.B.; funding acquisition, M.Y.F, S.I.B.
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through Research Group Project under grant number (R.G.P.1/119/40). Also authors extend their appreciation to the International Center for training and Advanced Researches (ICTAR–Egypt) for partial financial supporting this work through research groups.
The authors declare that they have no competing interests.
Journal of Cytology & Histology received 2476 citations as per Google Scholar report